
Organic Chemistry: Structure and Function
8th Edition
ISBN: 9781319079451
Author: K. Peter C. Vollhardt, Neil E. Schore
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 2.6, Problem 2.25TIY
(a)
Interpretation Introduction
Interpretation: The structure of
Concept introduction: As per IUPAC recommendations longest chain found in a continuous manner in a branched molecule is chosen as parent chain. Names of branches that occur commonly as side chains are listed below:
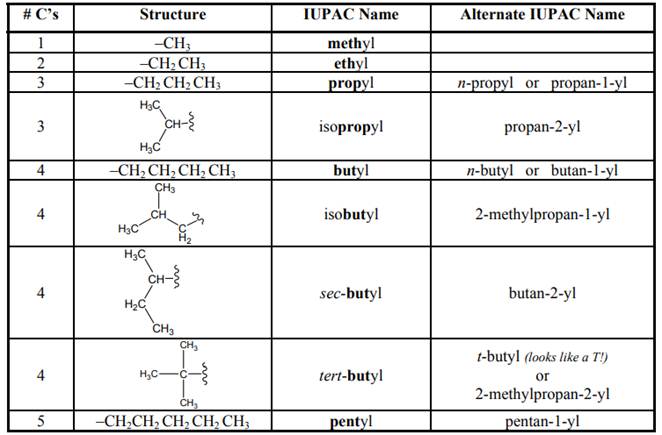
(b)
Interpretation Introduction
Interpretation: The structures corresponding to
Concept introduction: As per IUPAC recommendations longest chain found in a continuous manner in a branched molecule is chosen as parent chain. Names of branches that occur commonly as side chains are listed below:
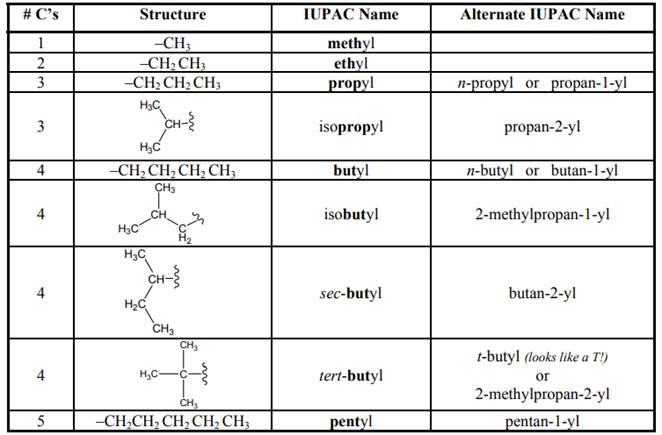
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. Octane, C8H18, has 18 different constitutional or chain isomers. One of them,
isooctane, is used as a standard in determining the octane rating of gasoline
a. Draw the structural formulas for at least ten chain isomers of octane.
b. Give the IUPAC name of each.
C. Which of the isomers that you have drawn has the highest boiling point?
Which has the lowest boiling point? Rationalize.
2. Which of the following structural formulas represent identical compounds
and which represent constitutional/structural isomers?
Identical compounds:
Constitutional isomers:
a). CH3CH2CHCH3
e). CH2CH2CHCH3
CH3
i). CH3-C-CI
ČI
CI
CI
CH3
CH2CI
b). CH3-C-CH3
f). CH3CH2CH2CH,CI
j). CICH2
CI
CH3
g). CICH,CHCH3
CH2CI
k). CH3-CH-CH3
CI
c). CH,CHCHCH3
CI
h). CH3CHCH2CH2CI
CH2CH3
1). CH3CHCI
d).
CI
CI
Petrol is a complex mix of light hydrocarbons. Petrol with octane number 95 contains 95% 2,2,4-trimethylpentane. This compound can be obtained by breaking up a large hydrocarbon molecule such as dodecane, C12H26 using a catalyst such as zeolite.
a) name the process where a large hydrocarbon molecule is broken into smaller molecules
b) write an equation for the breaking up of dodecane into 2,2,4-trimethylpentane if another branched hydrocarbon compound is formed at the same time.
c) what is the other hydrocarbon obtained ?
d) draw the structural formula of 2,2,4-trimethylpentane.
1. Octane, C8H18, has 18 different constitutional or chain isomers. One of them,
isooctane, is used as a standard in determining the octane rating of gasoline
a. Draw the structural formulas for at least ten chain isomers of octane.
b. Give the IUPAC name of each.
C. Which of the isomers that you have drawn has the highest boiling point?
Which has the lowest boiling point? Rationalize.
Chapter 2 Solutions
Organic Chemistry: Structure and Function
Ch. 2.1 - Prob. 2.1ECh. 2.1 - Prob. 2.2ECh. 2.1 - Prob. 2.3ECh. 2.1 - Prob. 2.5TIYCh. 2.1 - Prob. 2.6ECh. 2.1 - Prob. 2.7ECh. 2.2 - Prob. 2.8ECh. 2.3 - Prob. 2.9ECh. 2.3 - Prob. 2.10ECh. 2.3 - Prob. 2.11E
Ch. 2.3 - Prob. 2.12ECh. 2.3 - Prob. 2.13ECh. 2.3 - Prob. 2.14ECh. 2.3 - Prob. 2.15ECh. 2.3 - Prob. 2.17TIYCh. 2.3 - Prob. 2.19TIYCh. 2.5 - Prob. 2.20ECh. 2.6 - Prob. 2.21ECh. 2.6 - Prob. 2.22ECh. 2.6 - Prob. 2.23ECh. 2.6 - Prob. 2.25TIYCh. 2.7 - Prob. 2.26ECh. 2.9 - Prob. 2.28TIYCh. 2 - Prob. 31PCh. 2 - Prob. 32PCh. 2 - Prob. 33PCh. 2 - Prob. 34PCh. 2 - Prob. 35PCh. 2 - Prob. 36PCh. 2 - Prob. 37PCh. 2 - Prob. 38PCh. 2 - Prob. 39PCh. 2 - Prob. 40PCh. 2 - Prob. 41PCh. 2 - Prob. 42PCh. 2 - Prob. 43PCh. 2 - Prob. 44PCh. 2 - Prob. 45PCh. 2 - Prob. 46PCh. 2 - Prob. 47PCh. 2 - Prob. 48PCh. 2 - Prob. 49PCh. 2 - Prob. 50PCh. 2 - Prob. 51PCh. 2 - Prob. 52PCh. 2 - Prob. 53PCh. 2 - Prob. 54PCh. 2 - Prob. 55PCh. 2 - Prob. 56PCh. 2 - Prob. 57PCh. 2 - Prob. 58PCh. 2 - Prob. 59PCh. 2 - Prob. 60PCh. 2 - Prob. 61PCh. 2 - Prob. 62PCh. 2 - Prob. 63PCh. 2 - Prob. 64PCh. 2 - Prob. 65PCh. 2 - Prob. 66P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the difference in bonding and in the general molecular formula between an alkene and an alkane with the same number of carbon atoms?arrow_forwardWhat is the difference in bonding and in general molecular formula between an alkene and a cycloalkane with the same number of carbon atoms?arrow_forwardSelect those compounds that can be correctly called unsaturated and classify each one as an alkene or an alkyne: a.CH3CH2CH3f. b.CH3CH=CHCH3g. c.h.CH2=CHCH2CH3 d.i. e.arrow_forward
- a) Draw the structures of the following organic compounds. i) cis-pent-2-ene ii) o-difluorobenzene iii) 3-phenylhexanoic acid b) Determine the IUPAC name of the following organic compounds. H H₂C- CH- pat. CH -CH₂ CI CI CHỊU CÁCHỊ CHỊCH, -CH₂ CH₂ CH₂ H-N CH₂ CH₂ CH₂ CH₂ c) Determine and draw the structure of the organic product for each reaction below. Br A) + NH3 B) B) C=C-CH3 + HCI H Harrow_forwardExplain the substitution reaction of cyclopropane.arrow_forwardWhich of the following is the characteristic feature of all alkynes? a. the presence of one or more carbon-carbon double bonds. b. the presence of one or more triple bonds. c. the presence of at least one carbon-carbon double bond and at least one carbon-carbon triple bond. d. the presence of a ring system.arrow_forward
- 1. a. Draw the structures of the two isomers of dibromoethane. Name them by the IUPAC system. b. Draw out the structures of the two isomers of iodopropane. Name them by the IUPAC system. c. Draw the structures of the three isomers of dichloroethene. Name each one by the IUPAC system, indicating stereochemistry where relevant.arrow_forwardPart 4. Draw the structural formula and molecular formula. 2 - methyl – 3 – pentenearrow_forward4) Using structural diagrams, write balanced equations showing the following reactions. State the type of reaction that is occurring in each question and name each product that is formed. a) The reaction of methanol and oxygen gas. b) The reaction of 2-butene and hydrogen gas c) The reaction between 1-propanol and ethanoic acid d) The reaction between 1-amino-2-methyl pentane and propanoic acidarrow_forward
- Consider the following organic molecules: A) Butanone B) 3-methyl-3-pentanol C) 1,3-diethylcyclo-1-pentene i. Draw all three organic molecules. ii. Which molecule would you expect to have the highest boiling point? iii. Which molecule would undergo an addition reaction with H₂O? Draw the product that is formed and state its IUPAC name.arrow_forward1. Draw the structural formula of the following 1-bromo-4-methylhexane 2-brobutane Dichlorodiiodomethanearrow_forward5) Join four carbon atoms together by single bonds. Is it possible to do this in more than one way? Add hydrogen atoms to all the unused valences of carbon atoms. 6) What is the molecular formula of the hydrocarbon molecule? 7) What are the common names and IUPAC names of the isomers? 8) Draw the structural formulas of these compounds. 9) Draw different conformations (Newman projection) of straight chain containing four-carbon alkane. N f10 f11 N 53°F Cloudy f12 < C insearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License