
Organic Chemistry: Structure and Function
8th Edition
ISBN: 9781319079451
Author: K. Peter C. Vollhardt, Neil E. Schore
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 2, Problem 34P
Interpretation Introduction
Interpretation: The acid-base reactions with curved arrows should be identified.
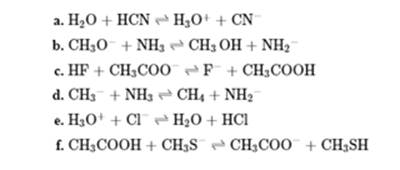
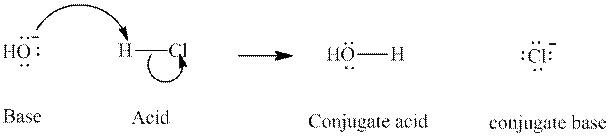
Concept introduction: In a typical acid-base reaction the fundamental principle is a lone pair of base reaches out for an acidic proton. Similar curved arrows are used to show the movement of electrons.. After deprotonation, the species left with a negative charge is referred to as the conjugate base of acid while the other with a positive charge is termed conjugate acid of given base. For example;
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has a weak conjugate base and the strong base has weak conjugate acid and vice-versa.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In the following acid - base reactions, a) Draw Lewis structures of the reactants and the products. b)
Determine which species are acting as electrophiles (acids) and which are acting as nucleophiles (bases).
c) Use the curved - arrow formalism to show the movement of electron pairs in these reactions and the
imaginary movement in the resonance hybrids of the products. d) Indicate which reactions are best
termed Brønsted-Lowry acid - base reactions i. CH3CHO + HCI--
> CH3CH2O
+ + Cl- ii. CH3CHO + OH- - -
> CH3CO-(OH) H
What are the products of the following reaction (drawing) ?
Draw the organic product of the Bronsted acid-base reaction.
Include all lone pairs and charges as appropriate. Ignore any
counterions.
H₂O*
Drawing
'H
Chapter 2 Solutions
Organic Chemistry: Structure and Function
Ch. 2.1 - Prob. 2.1ECh. 2.1 - Prob. 2.2ECh. 2.1 - Prob. 2.3ECh. 2.1 - Prob. 2.5TIYCh. 2.1 - Prob. 2.6ECh. 2.1 - Prob. 2.7ECh. 2.2 - Prob. 2.8ECh. 2.3 - Prob. 2.9ECh. 2.3 - Prob. 2.10ECh. 2.3 - Prob. 2.11E
Ch. 2.3 - Prob. 2.12ECh. 2.3 - Prob. 2.13ECh. 2.3 - Prob. 2.14ECh. 2.3 - Prob. 2.15ECh. 2.3 - Prob. 2.17TIYCh. 2.3 - Prob. 2.19TIYCh. 2.5 - Prob. 2.20ECh. 2.6 - Prob. 2.21ECh. 2.6 - Prob. 2.22ECh. 2.6 - Prob. 2.23ECh. 2.6 - Prob. 2.25TIYCh. 2.7 - Prob. 2.26ECh. 2.9 - Prob. 2.28TIYCh. 2 - Prob. 31PCh. 2 - Prob. 32PCh. 2 - Prob. 33PCh. 2 - Prob. 34PCh. 2 - Prob. 35PCh. 2 - Prob. 36PCh. 2 - Prob. 37PCh. 2 - Prob. 38PCh. 2 - Prob. 39PCh. 2 - Prob. 40PCh. 2 - Prob. 41PCh. 2 - Prob. 42PCh. 2 - Prob. 43PCh. 2 - Prob. 44PCh. 2 - Prob. 45PCh. 2 - Prob. 46PCh. 2 - Prob. 47PCh. 2 - Prob. 48PCh. 2 - Prob. 49PCh. 2 - Prob. 50PCh. 2 - Prob. 51PCh. 2 - Prob. 52PCh. 2 - Prob. 53PCh. 2 - Prob. 54PCh. 2 - Prob. 55PCh. 2 - Prob. 56PCh. 2 - Prob. 57PCh. 2 - Prob. 58PCh. 2 - Prob. 59PCh. 2 - Prob. 60PCh. 2 - Prob. 61PCh. 2 - Prob. 62PCh. 2 - Prob. 63PCh. 2 - Prob. 64PCh. 2 - Prob. 65PCh. 2 - Prob. 66P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. Use curved arrows to show electron movement in the reactant side and draw the product/s of the Lewis (nucleophile-electrophile) reaction. Draw in all lone pairs and charges where appropriate. acid-base -co +arrow_forwardAdd curved arrows to the following polar reactions to indicate the flow of electrons in each:arrow_forwardA benzene ring alters the reactivity of a neighboring group in the so-called “benzylic” position, similarly to how a double bond alters the reactivity of groups in the “allylic” position. Benzylic cations, anions, and radicals are all more stable than simple alkyl intermediates. a) Use resonance structures to show the delocalization of the positive charge, negative charge, and unpaired electron of the benzyl cation, anion, and radical.arrow_forward
- The following is generic depiction of a reaction using the curve arrow formalism. -D Which of these statements is not correct for this reaction? Electrons move from C to B. Electrons move from B to A. O In the products, a bond forms between C and B. O In the products, A would have a positive charge.arrow_forwardQuestion 43 of 50 A) SN1 Consider the reaction below. Predict which reaction type is likely to occur. NaH DMSO Heat Submit Tap here or pull up for additional resources OTSarrow_forwardWrite the product or products that will be formed as a result of the reactions given in each line below.arrow_forward
- ← Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the final product formed in this reaction. Include all lone pairs and charges as appropriate. Ignore inorganic byproducts. Problem 382 of 2 ེ་ ཆོས་རིག་ཆ་ Draw Intermediate 0° Darrow_forwardCircle aromatic compound(s)arrow_forwardUse curved arrows to show the most likely acid-base reaction between phenol and NaOH. a. Use pKa data to mark each curved arrow with a positive or negative energy change in pKa ,units. b. Calculate H for this reaction, and sketch an energy diagram showing H as an arrow onyour diagram.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning