
Organic Chemistry: Structure and Function
8th Edition
ISBN: 9781319079451
Author: K. Peter C. Vollhardt, Neil E. Schore
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 2, Problem 63P
Interpretation Introduction
Interpretation:The type of reaction represented by the below energy profile diagram should be chosen.
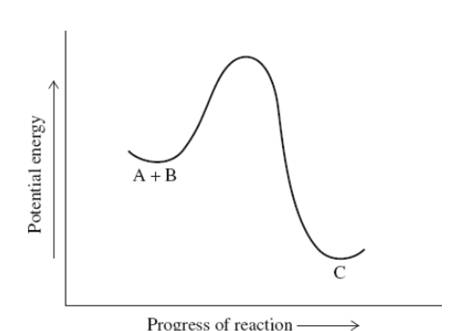
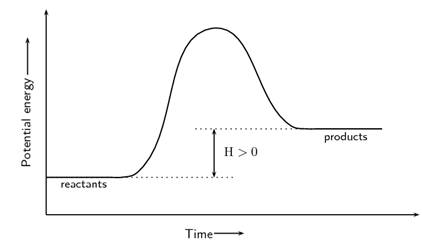
Concept introduction:The reactions that are accompanied by absorption of energy by substrates so as to form product are termed as endothermic reaction.The reaction profiles for such reaction are illustrated below.
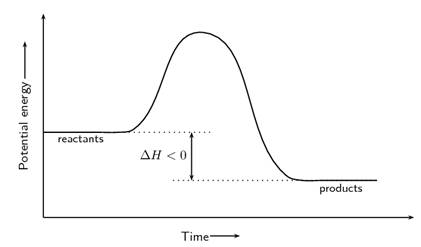
The reaction that liberates energy when reactants are converted to products is termed as exothermic reaction. The reaction profiles for such reaction are illustrated below.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A catalyst is
a.
A substance which increases the rate of a reaction by adding heat to it.
b.
A substance which increases the rate of a reaction without being used up in the reaction.
c.
An intermediate in a reaction which influences its rate.
d.
A solid which provides a "kick start" for the reaction, but is not affected by it.
Are the products or reactants of this reaction storing more energy in their chemical bonds?
Energy Diagram
Reaction Time (min)
a. Both storing the same
b. Products
c. No way to tell
d. Reactants
Potential Energy (kJ)
1. What is the main functional group of the organic molecule?*A. Aldehyde functional group
b. Carboxyl functional group
c. Hydroxyl functional group
d. Carbonyl functional group
2. What structure will characterize the intermediate in this reaction mechanism, after the leaving group is removed?*A. Change in conformation
b. Rearranged orbitals
c. Oxonium formation
d. Carbocation formation
Chapter 2 Solutions
Organic Chemistry: Structure and Function
Ch. 2.1 - Prob. 2.1ECh. 2.1 - Prob. 2.2ECh. 2.1 - Prob. 2.3ECh. 2.1 - Prob. 2.5TIYCh. 2.1 - Prob. 2.6ECh. 2.1 - Prob. 2.7ECh. 2.2 - Prob. 2.8ECh. 2.3 - Prob. 2.9ECh. 2.3 - Prob. 2.10ECh. 2.3 - Prob. 2.11E
Ch. 2.3 - Prob. 2.12ECh. 2.3 - Prob. 2.13ECh. 2.3 - Prob. 2.14ECh. 2.3 - Prob. 2.15ECh. 2.3 - Prob. 2.17TIYCh. 2.3 - Prob. 2.19TIYCh. 2.5 - Prob. 2.20ECh. 2.6 - Prob. 2.21ECh. 2.6 - Prob. 2.22ECh. 2.6 - Prob. 2.23ECh. 2.6 - Prob. 2.25TIYCh. 2.7 - Prob. 2.26ECh. 2.9 - Prob. 2.28TIYCh. 2 - Prob. 31PCh. 2 - Prob. 32PCh. 2 - Prob. 33PCh. 2 - Prob. 34PCh. 2 - Prob. 35PCh. 2 - Prob. 36PCh. 2 - Prob. 37PCh. 2 - Prob. 38PCh. 2 - Prob. 39PCh. 2 - Prob. 40PCh. 2 - Prob. 41PCh. 2 - Prob. 42PCh. 2 - Prob. 43PCh. 2 - Prob. 44PCh. 2 - Prob. 45PCh. 2 - Prob. 46PCh. 2 - Prob. 47PCh. 2 - Prob. 48PCh. 2 - Prob. 49PCh. 2 - Prob. 50PCh. 2 - Prob. 51PCh. 2 - Prob. 52PCh. 2 - Prob. 53PCh. 2 - Prob. 54PCh. 2 - Prob. 55PCh. 2 - Prob. 56PCh. 2 - Prob. 57PCh. 2 - Prob. 58PCh. 2 - Prob. 59PCh. 2 - Prob. 60PCh. 2 - Prob. 61PCh. 2 - Prob. 62PCh. 2 - Prob. 63PCh. 2 - Prob. 64PCh. 2 - Prob. 65PCh. 2 - Prob. 66P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw an energy diagram graph for an endothermic reaction where no catalyst is present. Then draw an energy diagram graph for the same reaction when a catalyst is present. Indicate the similarities and differences between the two diagrams.arrow_forwardIn general, what is the relationship between reaction rate and reactant concentration?arrow_forwardHow would you express the rate of the chemical reaction AB based on the concentration of Reactant A? Howwould that rate compare with the reaction rate based onthe Product B?arrow_forward
- A catalyst: a. Actually participates in the reaction b.changes the equilibrium concentration of the products c. Does not affect a reaction energy path d. always decreases rate of a reaction e. Increases activation energy for a reaction a or c???arrow_forwardHelp with reaction that matches the energy diagramarrow_forwardThe reaction diagram shown represents which type of reaction? Reaction Coordinate One-step, fast, exergonic a. One-step, fast, endergonic b. Two-step, fast, endergonic C. Two-step, slow, exergonic d. One-step, slow, exergonic е. Energyarrow_forward
- When all reactants were not completely converted to products after a stoichiometric amount of reactants were mixed is known as A. Non-reversible reaction B. Reversible reaction C. Rate of reaction D. First order reactionarrow_forwardSketch a qualitative reaction energy diagram for a chemical reaction with and without a catalyst. Assume the uncatalyzed reaction is endothermic. Note: Because the sketches are only qualitative, the energies in them don't have to be exact. They only have to have the right relationship to each other. For example, if one energy is less than another, that fact should be clear in your sketch. Uncatalyzed reaction Catalyzed reaction energy energy reactants products reactants Ea products reaction coordinate reaction coordinatearrow_forwardWhich of the following changes leads to a decrease in reaction rate? A. Increase the reactant concentration. B. Increase the frequency of collisions. C. Lower the product concentration. D. Increase reaction temperature. E. Increase the surface area for a reaction. F. Lower the frequency of collisions.arrow_forward
- When all reactants are converted to products after a stoichiometric amount of reactants were mixed is known as A. Non-reversible reaction B. Reversible reaction C. Rate of reaction D. First order reactionarrow_forwardWhy does a higher concentration make a reaction faster?arrow_forward2. Reaction I has a AG of 16.2 kJ/mol and Reaction 2 has a AG of -62.1 kJ/mol. Which statement is correct about these two reactions? A. Reaction 1 occurs faster. B. Reaction 2 occurs faster. C. Both reactions occur at the same rate. D. Reaction I will never occur. E. It is impossible to know which reaction occurs faster with this information. Answer:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License