
Concept explainers
(a)
Interpretation: The Newman projection for the rotation about the
Concept introduction: Various interconvertible forms that result from rotation around the
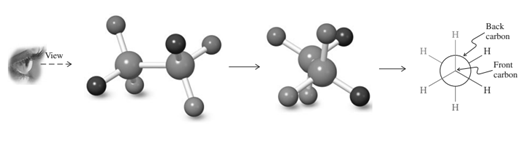
Thus in Newman projection of simple ethane molecule the “front”
(b)
Interpretation: The Newman projection for the rotation about the
Concept introduction: Various interconvertible forms that result from rotation around the
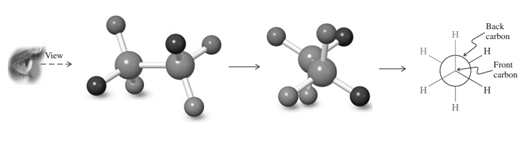
Thus in Newman's projection of simple ethane molecule the “front”
(c)
Interpretation: The Newman projection for the rotation about the
Concept introduction: Various interconvertible forms that result from rotation around the
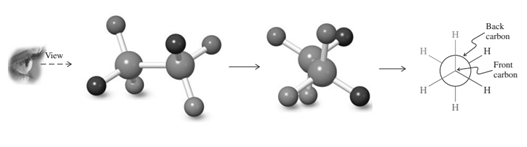
As rotation is carried out along
(d)
Interpretation: The Newman projection for the rotation about the
Concept introduction: Various interconvertible forms that result from rotation around the
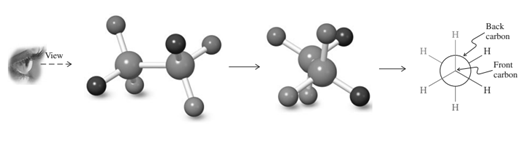
As rotation is carried out along
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Organic Chemistry: Structure and Function
- 4. (a) Draw a skeletal (line-bond) structure for 3,4-dimethylhexane. (b) Draw a sawhorse representation of any staggered conformation of this molecule looking down the carbon-3 to carbon-4 bond. (c) Draw a Newman projection looking down the carbon-3 to carbon-4 bond of the same conformation that you drew as a sawhorse representation.arrow_forwardDetermine the number of hydrogen atoms in each compound, given the number and type of nonhydrogen atoms it contains and its IHD.(a) Four carbon atoms; IHD = 0(b) Four carbon atoms; IHD =2(c) Three carbon atoms; two oxygen atoms; IHD = 1(d) Five carbon atoms; two chlorine atoms; one nitrogen atom; IHD = 3(e) One carbon atom; one nitrogen atom; IHD = 2(f) Six carbon atoms; two nitrogen atoms; one oxygen atom; three fluorine atoms; IHD = 4arrow_forwardConsider 1-bromopropane, CH3CH2CH2Br. (a) Draw a Newman projection for the conformation in which CH3 and -Br are anti (dihedral angle 180°). (b) Draw Newman projections for the conformations in which - CH3 and -Br are gauche (dihedral angles 60° and 300°). (c) Which of these is the lowest energy conformation? (d) Which of these conformations, if any, are related by reflection?arrow_forward
- Your chemistry professor draws a number of molecules on the board: (1) CH4 ; (2) H2C=CH2 ; (3) H2C=C=CH2 ; and (4) H2C=C=C=CH2. You muse about all the molecules that chemists draw on a two-dimensional board and wonder which ones are actually planar, existing basically as they appear on the board, and which ones are not plane but rather three-dimensional. Answer ALL of the following questions. What are the specific orbital overlaps (i.e., sp3-sp3) that are in each of the molecules? What are the bond angles for each central atom in each molecule? Which molecules are planar and which are non-planar?arrow_forwardYour chemistry professor draws a number of molecules on the board: (1) CH4 ; (2) H2C=CH2 ; (3) H2C=C=CH2 ; and (4) H2C=C=C=CH2. You muse about all the molecules that chemists draw on a two-dimensional board and wonder which ones are actually planar, existing basically as they appear on the board, and which ones are not plane but rather three-dimensional. Answer the following questions. What is the geometry and hybridization of the carbon in CH4? What is the geometry and hybridization of each central carbon atom in the remaining molecules? Draw each molecule showing the bonds and identify each bond in all the molecules as s or p. What are the specific orbital overlaps (i.e., sp3-sp3) that are in each of the molecules? What are the bond angles for each central atom in each molecule? Which molecules are planar and which are non-planar?arrow_forwardDraw the structures of 1,3-pentadiene and 1,4-pentadiene and label the carbons. Predict the trendin C-C single bond lengths in the two compounds.arrow_forward
- Name the following groups. CH3- CH3CH₂- CH3 CH3C- CH3 group group grouparrow_forwardName the following hydrocarbons: H;C CH3 (a) H,C=Ċ-Ċ=CH, H H (b) H;C-C=C-C=C-CH3 H H CH3 (c) H;C-C-CH,–CH3 CH3 CH, (d) H;C-C-CH3arrow_forward5-33 Draw chiral molecules that meet the following descriptions: (a) A chloroalkane, C5H11CI (b) An alcohol, C6H140 (c) An alkene, C6H12 (d) An alkane, C3H18arrow_forward
- • -- H (b) H,C 3-methylhexane CH, (c) B கூ CH, CH, HgCG; சீகூ H₂CH H 3-ethyl-1-methyloctane 3,3 diethyl pentane 5Å, Å,CH, W CH3 CH2 CH₂ W21 Su2... CH3 X -CH3 G b F E L carrow_forwardDraw an expanded structural formula of pent-1-en-3-yne/ CH3-CC-CH=CH2 and then label each carbon. Indicate the longest and shortest C-H bond and predict the C—C single bond that has the highest BDE(bond dissociation energy).arrow_forwardUsing Newman projections draw the most stable and least stable conformers of 3-methylhexane, viewed along the C2-C3 bond.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





