
(a)
Interpretation: The formula of conjugate acid of base dimethylamide should be written.
Concept introduction: In accordance with Bronsted definition, an acid act as proton donor and a base can act as proton acceptor. Thus, in a typical acid-base reaction, the fundamental principle is a lone pair of base reaches out for an acidic proton. Similarly, curved arrows are used for departing conjugate base. After deprotonation, the species left with negative charge is refers as conjugate base of acid while the other with positive charge is termed conjugate acid of given base. For example:
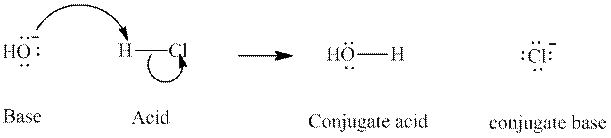
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has weak conjugate base and strong base has weak conjugate acid and vice-versa.
(b)
Interpretation: The formula of conjugate acid of sulfide should be written.
Concept introduction: In accordance with Bronsted definition, an acid act as proton donor and a base can act as proton acceptor. Thus, in a typical acid-base reaction, the fundamental principle is a lone pair of base reaches out for an acidic proton. Similarly, curved arrows are used for departing conjugate base. After deprotonation, the species left with negative charge is refers as conjugate base of acid while the other with positive charge is termed conjugate acid of given base. For example:
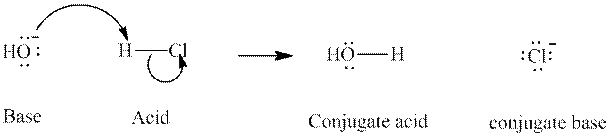
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has weak conjugate base and strong base has weak conjugate acid and vice-versa.
(c)
Interpretation: The formula of conjugate acid of ammonia should be written.
Concept introduction: In accordance with Bronsted definition, an acid act as proton donor and a base can act as proton acceptor. Thus, in a typical acid-base reaction, the fundamental principle is a lone pair of base reaches out for an acidic proton. Similarly, curved arrows are used for departing conjugate base. After deprotonation, the species left with negative charge is refers as conjugate base of acid while the other with positive charge is termed conjugate acid of given base. For example:
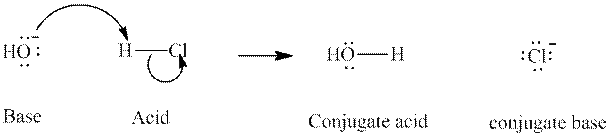
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has weak conjugate base and strong base has weak conjugate acid and vice-versa.
(d)
Interpretation: The formula of conjugate acid of acetone should be written.
Concept introduction: In accordance with Bronsted definition, an acid act as proton donor and a base can act as proton acceptor. Thus, in a typical acid-base reaction, the fundamental principle is a lone pair of base reaches out for an acidic proton. Similarly, curved arrows are used for departing conjugate base. After deprotonation, the species left with negative charge is refers as conjugate base of acid while the other with positive charge is termed conjugate acid of given base. For example:
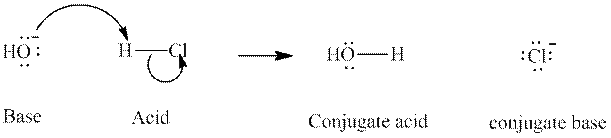
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has weak conjugate base and strong base has weak conjugate acid and vice-versa.
(e)
Interpretation: The formula of conjugate acid of
Concept introduction: In accordance with Bronsted definition, an acid act as proton donor and a base can act as proton acceptor. Thus, in a typical acid-base reaction, the fundamental principle is a lone pair of base reaches out for an acidic proton. Similarly, curved arrows are used for departing conjugate base. After deprotonation, the species left with negative charge is refers as conjugate base of acid while the other with positive charge is termed conjugate acid of given base. For example:
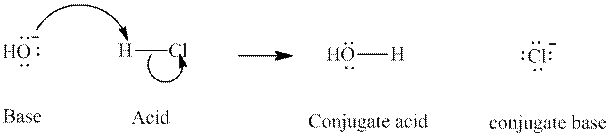
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has weak conjugate base and strong base has weak conjugate acid and vice-versa.
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Organic Chemistry: Structure and Function
- What is the freezing point of vinegar, which is an aqueous solution of 5.00% acetic acid, HC2H3O2, by mass (d=1.006g/cm3)?arrow_forwardThe ionization constant of lactic acid, CH,CH(OH)CO,H, an acid found in the blood after strenuous exercise, is 1.36 x 10-4. What is the concentration of hydronium ion in the solution of 0.102 M lactic acid? CH;CH(OH)CO,H (aq) + H,O (1) → H;O• (aq) + CH;CH(OH)CO, (aq) O 0.00372 M O 0.0165 M O 0.00549 M O 0.227 Marrow_forwardThe acid dissociation constant K, of alloxanic acid (HC,H;N,0,) is 2.24 x 10¬'. Calculate the pH of a 4.2 M solution of alloxanic acid. Round your answer to 1 decimal place. pH =arrow_forward
- 8) What is the pH of a 0.035 M HNO, solution? HNO3 (aq) + H,0 (1) H;O* (aq) + NO; (aq)arrow_forwardPredict whether aqueous solutions of the following substances are acidic, basic, or neutral and write hydrolysis equations for the acidic and basic solutions. (a) CsBr; (b) Al(NO3)3; (c) KCN; (d) CH3NH3Clarrow_forwardIn the following reactions, identify the Lewis acid and the Lewis base. (a) AlCl3 + Cl¯ → AICI, (b) CH;COOH(aq) + NH3(aq) → CH;COO (aq) + NH (aq) (c) Co³* (aq) + 6F (aq) → [CoF, j*¯ (aq)arrow_forward
- Which of the following reactions is an acid-base reaction? A) AGNO:(aq) + KF(aq) → AgF(s) + KNO:(aq) B) NH:(aq) + HF(aq) → NH:"(aq) + -F(aq) C) NO: (aq) + AI(s) → NH3(g) + AIO: (aq) D) CH:(g) + 202(g) - 2H:O(g) + CO:(g)arrow_forwardUse the Referenc The pOH of an aqueous solution of 0.313 M acetylsalicylic acid (aspirin), HC9H¬O4, isarrow_forward(i) Define pH in words. The strong acid HClaq has a pH value of 1, use the following equation for a strong acid: HClaq à H+aq + Cl-aq and convert the following expression to deduce the hydrogen ion concentration: pH = -log10 [H+] (ii) Use the above expression to deduce the pH of HCl (aq) given the concentration of the acid to be 4.5 mol/dm3arrow_forward
- (i) Define pH in words. The strong acid HClaq has a pH value of 1, use the following equation for a strong acid: HClaq à H+aq + Cl-aq and convert the following expression to deduce the hydrogen ion concentration: pH = -log10 [H+] (ii) Use the above expression to deduce the pH of HCl (aq) given the concentration of the acid to be 4.5 mol/dm3 pH =arrow_forwardThe pOH of an aqueous solution of 0.591 M phenol (a weak acid), C,H5OH, isarrow_forwardThe base protonation constant K, of azetidine (C,H,NH) is 1.5 × 10 Calculate the pH of a 1.1 M solution of azetidine at 25 °C. Round your answer to 1 decimal place. pH = |arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

