
Concept explainers
(a)
Interpretation: Structures of the five isomeric hexanes should be drawn.
Concept introduction: Molecules that possess identical molecular formula but vary in the manner of their connectivity of the bonds are referred as structural isomers. These are essentially found in
For instance, smallest branched alkane possible is
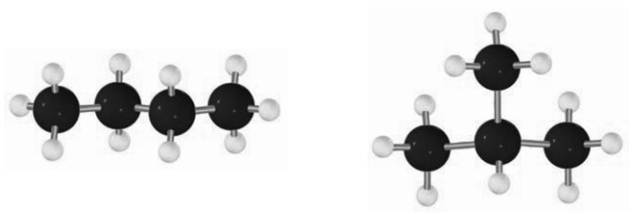
The two compounds infact constitute a pair of constitutional isomers. These kinds of isomers are formed by cleavage and replacement of bonds, unlike the conformational isomers that result due to rotation around the bond.
(b)
Interpretation: Structures of all the possible next higher and lower homologs of
Concept introduction: Each member of a particular functional group series differs from the next lower by the addition of a methylene group
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Organic Chemistry: Structure and Function
- Draw all possible structure(s) and give the IUPAC systematic name(s) of an alkane or cycloalkane with the formula C8H18 that has only primary hydrogen atoms...arrow_forwardWhy do alkynes not didplay a cis- and trans- isomers?arrow_forwardEncricle the functional groups in the followingf compounds, label each with the letters indicated and indentify the class in which the functional group may be foundarrow_forward
- Among alkenes, alkynes, and aromatic hydrocarbons, onlyalkenes exhibit cis-transisomerism. Why don’t the others?arrow_forwardWrite the molecular formula of the 2nd and the 3rd member of the homologous series whose first member is methane.arrow_forwardWhat is the iupac names of the followings structures ? Should they have their names cis and trans?arrow_forward
- Quiana whose structure is shown below is a synthetic fabric that feels very much like silk. What are the structures of the two monomers that are used to make Quiana?arrow_forward1,3-dimethylcyclohexane and Cl2 forms what?arrow_forwardA. Which class/classes* of hydrocarbons is/are reactive to Br2 in CH2Cl2 ONLY in the presence of light? b. Which class/classes of hydrocarbons is/are reactive to Br2 in CH2Cl2 WITH OR WITHOUT light? c. Which class/classes of hydrocarbons is/are not reactive to Br2 in CH2Cl2 BOTH in the presence and absence of light?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

