
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
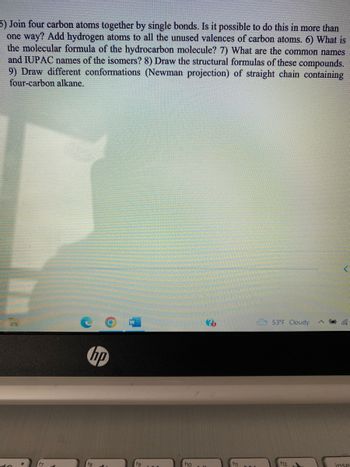
Transcribed Image Text:5) Join four carbon atoms together by single bonds. Is it possible to do this in more than
one way? Add hydrogen atoms to all the unused valences of carbon atoms. 6) What is
the molecular formula of the hydrocarbon molecule? 7) What are the common names
and IUPAC names of the isomers? 8) Draw the structural formulas of these compounds.
9) Draw different conformations (Newman projection) of straight chain containing
four-carbon alkane.
N
f10
f11
N
53°F Cloudy
f12
<
C
inse
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Additional Questions 1. Draw and name all isomers of the alkane C6H14- 2. Give the correct IUPAC name for each of the four compounds: CH3 a) CH3-CH—CH,CHCH3 CH₂ CH3 c) CH3-CH₂-CH-CH3 CH3 c. 2,2,3-trimethylhexane d. 4-ethyl-2,2-dimethylhexane CI b) CH3-C- -CH₂CH3 CH3 3. Draw the structural formulas for the following: a. 2-chloropentane b. 3-chloro-3-methylpentane G₂H5 d) CHỖ CH,CHCHCH3 CH(CH3)2arrow_forwardPlease helparrow_forwardCan you please do the last three rows of this table. The trichloroethane, ethane, and bromoethyne?arrow_forward
- Why do alkanes, alkenes, and alkynes have a relatively low boiling point? O They have no large intermolecular forces holding them together. O They have no diple moments or polarity. O They have a lot of electron density in a very small amout of space.arrow_forwardExplain why two different straight-chain alkanes could not be constitutional isomers.arrow_forwardOn a blank sheet of paper draw the structures for the following namesarrow_forward
- When numbering the carbons in alkenes or alkynes, Question 7 options: The carbon that contains the first branch is numbered carbon 1. It does not matter where you begin numbering. The location of the multiple bond takes precedence There is no system in place for this.arrow_forward3) Draw and name an alcohol that has 3 different substituents, one should be an alkene. You cannot have the same example as someone else.arrow_forwardWhich of the following straight-chain alkanes has the lowest boiling point? A) nonane B) heptane C) pentane D) decane E) ethanearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY