
Organic Chemistry: Structure and Function
8th Edition
ISBN: 9781319079451
Author: K. Peter C. Vollhardt, Neil E. Schore
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 2, Problem 65P
Interpretation Introduction
Interpretation: The kind of Newman projection of the conformer of butane shown in below structural representation should be identified.
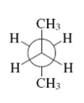
Concept introduction: Various interconvertible forms that results from rotation around the bind in ethane are termed as conformer. To depict such conformation Newman projections are written. These are obtained by conversion of hashed-wedged line structure simply when molecule is viewed in a plane along the as illustrated below.
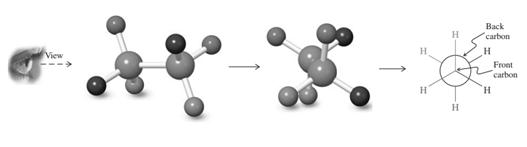
Thus in Newman's projection of simple ethane molecule, the “front” is depicted as intersection of three bonds with three attached . The other three bonds connected to the large circle corresponding to “back” carbon in bond.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used hand raiting
Diferencie entre semiconductores extrínsecos de tipo n y semiconductores extrínsecos de tipo p.
None
Chapter 2 Solutions
Organic Chemistry: Structure and Function
Ch. 2.1 - Prob. 2.1ECh. 2.1 - Prob. 2.2ECh. 2.1 - Prob. 2.3ECh. 2.1 - Prob. 2.5TIYCh. 2.1 - Prob. 2.6ECh. 2.1 - Prob. 2.7ECh. 2.2 - Prob. 2.8ECh. 2.3 - Prob. 2.9ECh. 2.3 - Prob. 2.10ECh. 2.3 - Prob. 2.11E
Ch. 2.3 - Prob. 2.12ECh. 2.3 - Prob. 2.13ECh. 2.3 - Prob. 2.14ECh. 2.3 - Prob. 2.15ECh. 2.3 - Prob. 2.17TIYCh. 2.3 - Prob. 2.19TIYCh. 2.5 - Prob. 2.20ECh. 2.6 - Prob. 2.21ECh. 2.6 - Prob. 2.22ECh. 2.6 - Prob. 2.23ECh. 2.6 - Prob. 2.25TIYCh. 2.7 - Prob. 2.26ECh. 2.9 - Prob. 2.28TIYCh. 2 - Prob. 31PCh. 2 - Prob. 32PCh. 2 - Prob. 33PCh. 2 - Prob. 34PCh. 2 - Prob. 35PCh. 2 - Prob. 36PCh. 2 - Prob. 37PCh. 2 - Prob. 38PCh. 2 - Prob. 39PCh. 2 - Prob. 40PCh. 2 - Prob. 41PCh. 2 - Prob. 42PCh. 2 - Prob. 43PCh. 2 - Prob. 44PCh. 2 - Prob. 45PCh. 2 - Prob. 46PCh. 2 - Prob. 47PCh. 2 - Prob. 48PCh. 2 - Prob. 49PCh. 2 - Prob. 50PCh. 2 - Prob. 51PCh. 2 - Prob. 52PCh. 2 - Prob. 53PCh. 2 - Prob. 54PCh. 2 - Prob. 55PCh. 2 - Prob. 56PCh. 2 - Prob. 57PCh. 2 - Prob. 58PCh. 2 - Prob. 59PCh. 2 - Prob. 60PCh. 2 - Prob. 61PCh. 2 - Prob. 62PCh. 2 - Prob. 63PCh. 2 - Prob. 64PCh. 2 - Prob. 65PCh. 2 - Prob. 66P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Shown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H. H. +N=C H H H Cl: Click and drag to start drawing a structure. : ? g B S olo Ar B Karrow_forwardDon't used hand raitingarrow_forwardS Shown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H H = HIN: H C. :0 H /\ H H Click and drag to start drawing a structure. ×arrow_forward
- Part II. two unbranched ketone have molecular formulla (C8H100). El-ms showed that both of them have a molecular ion peak at m/2 =128. However ketone (A) has a fragment peak at m/2 = 99 and 72 while ketone (B) snowed a fragment peak at m/2 = 113 and 58. 9) Propose the most plausible structures for both ketones b) Explain how you arrived at your conclusion by drawing the Structures of the distinguishing fragments for each ketone, including their fragmentation mechanisms.arrow_forwardPart V. Draw the structure of compound tecla using the IR spectrum Cobtained from the compound in KBr pellet) and the mass spectrum as shown below. The mass spectrum of compound Tesla showed strong mt peak at 71. TRANSMITTANCE LOD Relative Intensity 100 MS-NW-1539 40 20 80 T 44 55 10 15 20 25 30 35 40 45 50 55 60 65 70 75 m/z D 4000 3000 2000 1500 1000 500 HAVENUMBERI-11arrow_forwardTechnetium is the first element in the periodic chart that does not have any stable isotopes. Technetium-99m is an especially interesting and valuable isotope as it emits a gamma ray with a half life ideally suited for medical tests. It would seem that the decay of technetium should fit the treatment above with the result In(c/c) = -kt. The table below includes data from the two sites: http://dailymed.nlm.nih.gov/dailymed/druginfo.cfm?id=7130 http://wiki.medpedia.com/Clinical: Neutrospec_(Technetium_(99m Tc)_fanolesomab). a. b. C. Graph the fraction (c/c.) on the vertical axis versus the time on the horizontal axis. Also graph In(c/c.) on the vertical axis versus time on the horizontal axis. When half of the original amount of starting material has hours fraction remaining disappeared, c/c = ½ and the equation In(c/c.) = -kt becomes In(0.5) = -kt1/2 where t₁₂ is the half life (the time for half of the material to decay away). Determine the slope of your In(c/c.) vs t graph and…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License


