
Concept explainers
(a)
Interpretation: The energy associated with single
Concept introduction: Various interconvertible forms that result from rotation around the
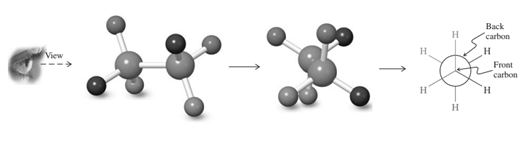
For example, energetics of rotation about the
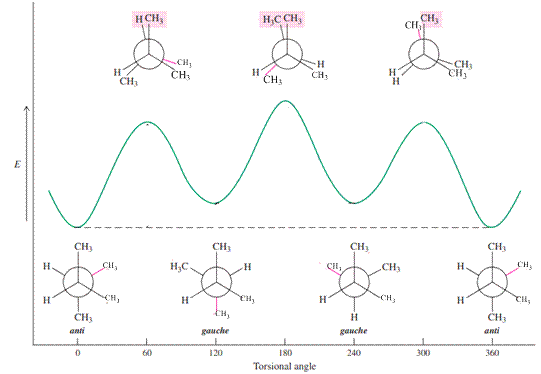
Because of relatively more bulkiness in the system compared to butane, the energy differences between the anti and all other conformations will increase, with the greatest increase at
(b)
Interpretation: The energy associated with single
Concept introduction: Various interconvertible forms that result from rotation around the
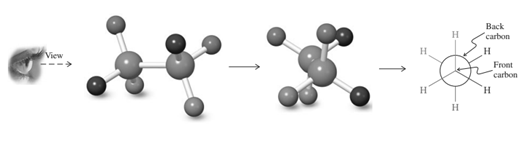
For example energetics of rotation about the
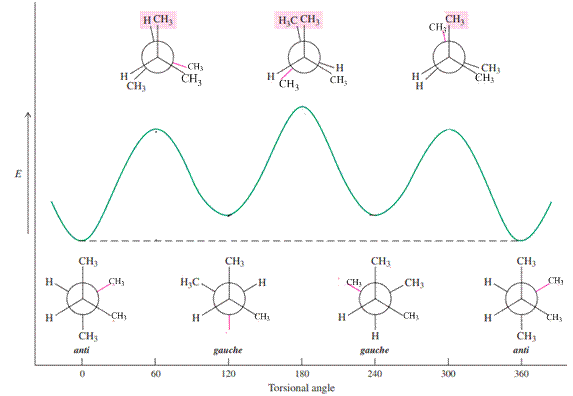
(c)
Interpretation: The energy associated with single methyl-methyl eclipsed interaction should be determined.
Concept introduction: Various interconvertible forms that result from rotation around the
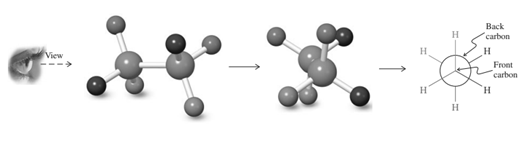
For example energetics of rotation about the
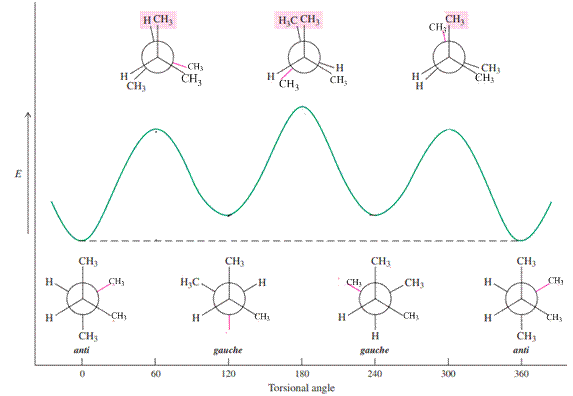
(d)
Interpretation: The energy associated with single methyl-methyl gauche interaction should be determined.
Concept introduction: Various interconvertible forms that result from rotation around the
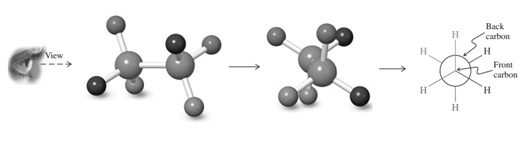
For example energetics of rotation about the
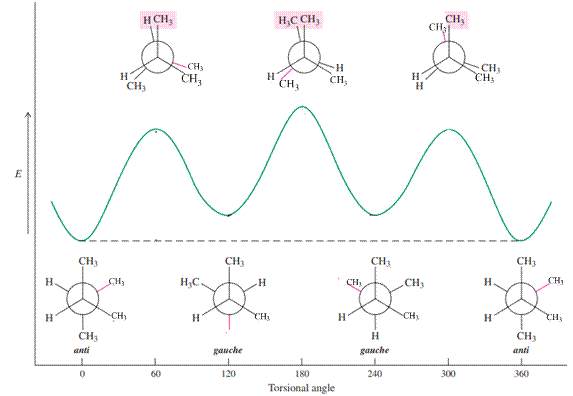
Because of relatively more bulkiness in the system compared to butane, the energy differences between the anti and all other conformations will increase, with the greatest increase at
Trending nowThis is a popular solution!

Chapter 2 Solutions
Organic Chemistry: Structure and Function
- Which of the following Newman projections is NOT a conformational isomer of 1,2-dibromoethane? Br H BrH Br H Br Br B.H- D. H- A. С. H- -Br -H- Br H. H Br HH H H H Harrow_forward9. Rank the C-H bond strength of the indicated hydrogen atoms in the following molecules (strongest to weakest C-H bond): B H. C =C _H ii 10. Rank the relative stability of the most stable conformation that the following cyclohexane isomers can adopt (most stable isomer to least stable isomer): B i iiarrow_forward||| O PRINCIPLES OF ORGANIC CHEMISTRY Classifying organic reactions Classify each of the following as either a substitution, elimination, or addition reaction. OH CH3 C CH3 O-CH3 Explanation - CH3 CH2=C—CH3 + H2O. CH3 CH₂ CH2-CH₂-OH- CH3 _용- CH3-C-CH₂-C-CH3 + H2. CH3 H* CH₂-0-0-CH CHy-O-C–CH,—CH, +H,O Check 8-CH₂ CH3-C-CH3 + HO-CH3 FEB 26 H* H' Pd/C H* CH3 CH3 C CH3 OH CH3–CH2CH=CH2 + H2O OH CH3 www-awu.aleks.com CH3-CH-CH₂-C-CH3 - CH3 8 CH₂ HỌ–CCH,CH, Á HO-CH, tv substitution O elimination O addition O substitution O elimination addition O substitution O elimination addition O substitution O elimination O addition O substitution O elimination O addition © 2023 McGraw Hill LLC. All Rights Reserved. A Fo C X Terms of Usarrow_forward
- Give one possibility for the mystery reactant R in this organic reaction: CH3 R + H2O CH3 CH2 C-CH-CH2-CH3 OH CH3 Specifically, in the drawing area below draw the condensed structure of what R might be. There may be more than one reasonable answer. Click anywhere to draw the first atom of your structure. ☑ : ☐ كarrow_forwardIncreased substitution around a bond leads to increased strain. Take the four substituted butanes listed below, for example. For each compound, sight along the C2-C3 bond and draw Newman projections of the most stable and least stable conformations. Use the data in Table 3-5 to assign strain-energy values to each conformation. Which of the eight conformations is most strained? Which is least strained? (a) 2-Methylbutane (b) 2,2-Dimethylbutane (c) 2,3-Dimethylbutane (d) 2,2,3-Trimethylbutanearrow_forwardFrom the data in Figure 4-12 and Table 4-1, estimate the percentages of molecules that have their substituents in an axial orientation for the following compounds: (a) Isopropylcyclohexane (b) Fluorocyclohexane (c) Cyclohexanecarbonitrile, C6H11CNarrow_forward
- Add a + or above each curved arrow in Figure 4.11 to show the sign of the energy change.arrow_forwardAccording to the conventions above, what is the sign ( + or ) of the P.E. change (H) for Rxn 3?arrow_forwardModify the structure of cyclohexane to show 1,1-dimethylcyclohexane. H₂C 1 H₂C CH₂ CH₂ Modify the structure of cyclohexane to show 1,3-dimethylcyclohexane. H₂C | H₂C UI CH₂ 1 CH₂arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

