
Concept explainers
Interpretation: The expected potential-energy diagram for the rotation about the 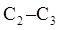 bond in
bond in 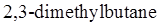 should be drawn along with Newman projections of each staggered and eclipsed conformation.
should be drawn along with Newman projections of each staggered and eclipsed conformation.
Concept introduction: Various interconvertible forms that results from rotation around the 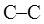 bond in ethane are termed as conformer. To depict such conformation Newman projections are written. These are obtained by conversion of hashed-wedged line structure simply when molecule is viewed in a plane along the
bond in ethane are termed as conformer. To depict such conformation Newman projections are written. These are obtained by conversion of hashed-wedged line structure simply when molecule is viewed in a plane along the 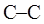 as illustrated below:
as illustrated below:
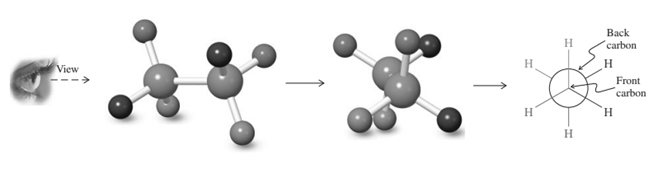
Thus in Newman projection of simple ethane molecule the “front” 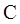 is depicted as intersection of three bonds with three attached
is depicted as intersection of three bonds with three attached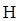 The other three bonds connect to the large circle corresponding to “back” carbon in
The other three bonds connect to the large circle corresponding to “back” carbon in  bond.
bond.
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Organic Chemistry: Structure and Function
- Consider 1-bromo-2-methylpropane and draw the following. (a) The staggered conformation(s) of lowest energy (b) The staggered conformation(s) of highest energyarrow_forwardmyo-Inositol, one of the isomers of 1,2,3,4,5,6-hexahydroxycyclohexane, acts as a growth factor in both animals and microorganisms. Draw the most stable chair conformation of myo-inositol.arrow_forwardFollowing is a planar hexagon representation for one isomer of 1,2,4-trimethylcyclohexane. Draw the alternative chair conformations of this compound and state which of the two is more stable.arrow_forward
- Following is a planar hexagon representation of L-fucose, a sugar component of the determinants of the A, B, O blood group typing. For more on this system of blood typing, see Chemical Connections: A, B, AB, and O Blood Group Substances in Chapter 25. (a) Draw the alternative chair conformations of L-fucose. (b) Which of them is more stable? Explain.arrow_forwardWhich conformation in the picture attached represents the most stable ECLIPSED conformation of 2,3-dimethylbutane along the C2-C3 bond?arrow_forwardWhich conformation is the most STABLE STAGGERED conformation of 2,3-dimethylbutane along the C2-C3 bond?arrow_forward
- Draw Newman projections, in order of decreasing energy, for all staggered conformations of 1,2- dibromooethane formed by rotation of the carbon-carbon bond from 0° to 180°.arrow_forwardConformational studies on ethane-1,2-diol (HOCH2¬CH2OH) have shown the most stable conformation about the central C¬C bond to be the gauche conformation, which is 9.6 kJ/mol (2.3 kcal/mol) more stable than the anti conformation. Draw Newman projections of these conformers, and explain this curious result.arrow_forwardDraw a potential-energy diagram for rotation about the C@2¬C@3 bond of pentane through 360°, starting with the least stable conformer.arrow_forward
- looking along C2-C3 draw Newman projections of the most stable conformation for 2,3,4-trimethyhexane. Explain your answer literally step by step.arrow_forwardDraw all three staggered conformations for the following compound, viewed along the C₂-C3 bond. Determine which conformation is the most stable, taking into account gauche interactions and hydrogen-bonding interactions. (J. Phys. Chem. A 2001, 105, 6991-6997) Provide a reason for your choice by identifying all of the interactions that led to your decision. H₂C OH C₂ HO CH3 Which of the following is the most stable staggered conformer? Me Me *** Conformer B Me- Me Conformer A Me Conformer C Me Conformer B because the anti OH groups avoid hydrogen bonding, which is a destabilizing effect. Conformer A because there are only two gauche interactions, and hydrogen bonding between the two OH groups is a stabilizing effect. Conformer C because there is no steric strain for Me-OH gauche interactions and hydrogen bonding between the two OH groups is a stabilizing effect. Conformer B because the large OH groups are anti to each other.arrow_forwardConsider 1-bromopropane, CH3CH2CH2Br. (a) Draw a Newman projection for the conformation in which CH3 and -Br are anti (dihedral angle 180°). (b) Draw Newman projections for the conformations in which - CH3 and -Br are gauche (dihedral angles 60° and 300°). (c) Which of these is the lowest energy conformation? (d) Which of these conformations, if any, are related by reflection?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

