
Organic Chemistry: Structure and Function
8th Edition
ISBN: 9781319079451
Author: K. Peter C. Vollhardt, Neil E. Schore
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 8, Problem 57P
Interpretation Introduction
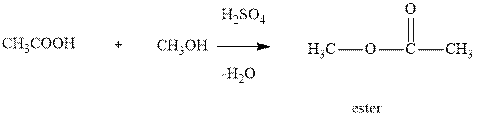
Interpretation:The synthesis of
Concept introduction:Ester is a compound formed by condensation between one equivalent of alcohol and one equivalent of
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Ketones and aldehydes react with sodium acetylide (the sodium salt of acetylene) to give alcohols, as shown in the following example:
R, OH
1. HC=C: Na*
2. Hзо
C.
R2
R2
HC
Draw the structure of the major reaction product when the following compound reacts with sodium acetylide, assuming that the reaction takes preferentially from the Re face of the
carbonyl group.
Use the wedge/hash bond tools to indicate stereochemistry where it exists.
You do not have to explicitly draw H atoms.
If a group is achiral, do not use wedged or hashed bonds on it.
4-pyranone will readily undergo an acid-base reaction.
Identify the reaction conditions that will result in the
formation of an aromatic product. Then, draw the
aromatic resonance product structure.
Include all lone pairs in your structure. Ignore inorganic
byproducts.
H3O+
Provide a mechanism for the following reaction. Show each step in your mechanism and use
curved arrows to show the movement of electron pairs. Hint: the first step is a proton
transfer; second step, nucleophilic attack.
OCH3
H,SO4
CH;OH
Chapter 8 Solutions
Organic Chemistry: Structure and Function
Ch. 8.1 - Prob. 8.1ECh. 8.1 - Prob. 8.2ECh. 8.3 - Prob. 8.4TIYCh. 8.3 - Prob. 8.5ECh. 8.3 - Prob. 8.6ECh. 8.4 - Prob. 8.7ECh. 8.5 - Prob. 8.8ECh. 8.5 - Prob. 8.9ECh. 8.5 - Prob. 8.10ECh. 8.5 - Prob. 8.11E
Ch. 8.5 - Prob. 8.12ECh. 8.6 - Prob. 8.14TIYCh. 8.7 - Prob. 8.15ECh. 8.7 - Prob. 8.16ECh. 8.8 - Prob. 8.18TIYCh. 8.8 - Prob. 8.19ECh. 8.8 - Prob. 8.21TIYCh. 8 - Prob. 24PCh. 8 - Prob. 25PCh. 8 - Prob. 26PCh. 8 - Prob. 27PCh. 8 - Prob. 28PCh. 8 - Prob. 29PCh. 8 - Prob. 30PCh. 8 - Prob. 31PCh. 8 - Prob. 32PCh. 8 - Prob. 33PCh. 8 - Prob. 34PCh. 8 - Prob. 35PCh. 8 - Prob. 36PCh. 8 - Prob. 37PCh. 8 - Prob. 38PCh. 8 - Prob. 39PCh. 8 - Prob. 40PCh. 8 - Prob. 41PCh. 8 - Prob. 42PCh. 8 - Prob. 43PCh. 8 - Prob. 44PCh. 8 - Prob. 45PCh. 8 - Prob. 46PCh. 8 - Prob. 47PCh. 8 - Prob. 48PCh. 8 - Prob. 49PCh. 8 - Prob. 50PCh. 8 - Prob. 51PCh. 8 - Prob. 52PCh. 8 - Prob. 53PCh. 8 - Prob. 54PCh. 8 - Prob. 55PCh. 8 - Prob. 56PCh. 8 - Prob. 57PCh. 8 - Prob. 58PCh. 8 - Prob. 59PCh. 8 - Prob. 60PCh. 8 - Prob. 61PCh. 8 - Prob. 62PCh. 8 - Prob. 63PCh. 8 - Prob. 64PCh. 8 - Prob. 65PCh. 8 - Prob. 66P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Recalling the reactions of alcohols from Chapter 10, show how to synthesize each compound from an organohalogen compound and an oxirane, followed by a transformation of the resulting hydroxyl group to the desired oxygen-containing functional group.arrow_forwardCriteria for satisfactory score Reactants, products, and reagents that complete a reaction scheme must specific compounds, not generic categories. Reagents and structures must be valid Lewis structures. Tasks 1. Complete the synthetic sequences by drawing products/substrates/reagents in empty spaces in reactions below. 2. Draw the mechanism of tautomerization (line 3).arrow_forwardThe reaction shown below is carried out in two stages. In the first, the two reactants are combined in tetrahydrofuran (THF) solvent to form a neutral adduct. Aqueous acid is then added to hydrolyze this initial adduct, giving the final product. N(CH3)2 I. H a For the reaction shown above, draw the major organic product having at least one carbonyl group. • You do not have to consider stereochemistry. Pis 85 1. THF 2. H3O+ n [ ]#arrow_forward
- Draw the principal organic product for the reaction of 2-bromohexane with lithium in diethyl ether, followed by formaldehyde in diethyl ether, and then followed by dilute acid. Click and drag to start drawing a structure. X A G Parrow_forwardDraw the organic product you would expect to isolate from the nucleophilic substitution reaction between the molecules shown. Note: You do not need to draw any of the side products of the reaction, only the substitution product. + H₂O Cl + ☑ 5 C Click and drag to start drawing a structure.arrow_forward4. For this one, you'll need to know that periodic acid, H5I06, cleaves diols to aldehydes and ketones. CO OHarrow_forward
- Complete the following reaction scheme. Keep in mind that the sequence starts with an alkene or alcohol of eight carbons or less. HBr B Mg diethyl ether с 1. D 2. H₂O+ OHarrow_forwardAcid-catalyzed dehydration of secondary and tertiary alcohols proceeds through an E1 mechanism. The first step is the protonation of the alcohol oxygen to form an oxonium ion. Dehydration of 3-methyl-2-butanol forms one major and two minor organic products. Draw the structures, including hydrogen atoms, of the three organic products of this reaction. н н :бн н Нас. Нас. CHз CH3 ČH3 CH3 3-methyl-2-butanol an oxonium ion Major Product Minor Product Minor Productarrow_forwardIn this chapter, we studied the mechanism of the acid-catalyzed hydration of an alkene. The reverse of this reaction is the acid-catalyzed dehydration of an alcohol. OH H,SO, CH,-CH-CH, CH-CH=CH, + H,O 2-Propanol (Isopropyl alcohol) Propene Propose a mechanism for the acid-catalyzed dehydration of 2-propanol to propene.arrow_forward
- Show how 1-propanol can be converted into the following compounds by means of a sulfonate ester:arrow_forwardDraw structures of compounds that fit the following descriptions: a) An , unsaturated ketone, C9H8O b) An diketone c) An aromatic ketone, C9H10O d) A diene aldehyde, C7H8Oarrow_forwardA postgraduate student wanted to synthesized two carbonyl compounds known as compounds M and N using oxidation of alcohol. However, these carbonyl compounds should have more than 6 carbon atoms. Draw two (2) possible structural formulae for compounds M and N. How would you distinguish between compounds M and N?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY