
Concept explainers
(a)
Interpretation: The synthesis of
Concept introduction: Retrosynthetic analysis involves transformation of target molecule into simple precursor that can be used as substrate for the synthesis of target molecule. The bonds broken generate molecules called synthons or synthetic equivalent. The latter term is designated to indicate their use as substrate in synthesis. For instance, the synthons generated in phenylacetic acid are given as follows:
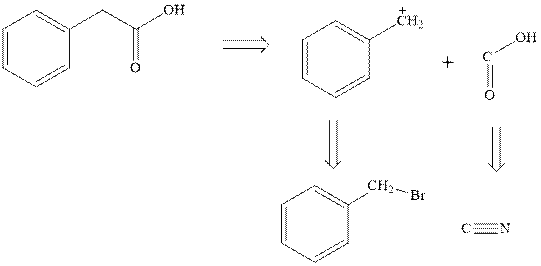
The best approach to retrosynthetic analysis is to look for easily available reagents such as Grignard reagents.
(b)
Interpretation: The synthesis of
Concept introduction:Retrosynthetic analysis involves transformation of target molecule into simple precursor that can be used as substrate for the synthesis of target molecule. The bonds broken generate molecules called synthons or synthetic equivalent. The latter term is designated to indicate their use as substrate in synthesis. For instance, the synthons generated in phenylacetic acid are given as follows:
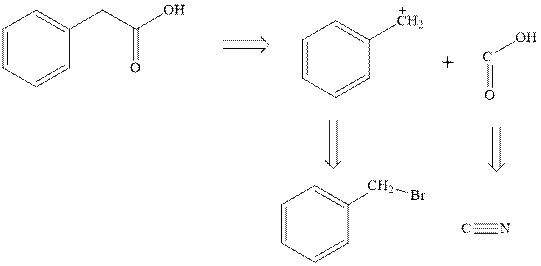
The best approach to the retrosynthetic analysis is to look for easily available reagents such as Grignard reagents.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry: Structure and Function
- Chlorination of 2-butanone yields two isomeric products, each having the molecular formula C4H7ClO. (a) What are these two compounds? (b) Write structural formulas for the enol intermediates that lead to each of these compounds. (c) Using curved arrows, show the flow of electrons in the reaction of each of the enols with Cl2.arrow_forward(b) 3-methyl-2-butanol reacts with concentrated sulphuric acid to form 2-methyl-2- butene. Write the mechanism for the reaction.arrow_forward(a) Why is the following reaction a poor method for the synthesis of t-butyl propyl ether? (b) What would be the major product from this reaction? (c) Propose a better synthesis of r-butyl propyl ether. ÇH, does not give ÇH, CH,CH,CH,-O: *Na + CH,-C-Br > CH,-C-o-CH,CH,CH, CH, CH, t-butyl propyl ether sodium propoxide 1-butyl bromidearrow_forward
- Write a reaction that could be carried out to synthesize 1-(4-methylphenyl)ethanol (reactants will be a combination of Grignard reagent and carbonyl compound).arrow_forwardHow could you convert each of the following compounds into butanoic acid? Write each step showing all reagents. (a) 1-Butanol (b) 1-Bromobutane (c) 1-Butenearrow_forwardGive reasons for the following: (i) p-nitrophenol is more acidic than p-methylphenol. (ii) Bond length of C—O bond in phenol is shorter than that in methanol. (iii) (CH3)3C—Br on reaction with sodium methoxide (Na+ _OCH3) gives alkene as the main product and not an ether.arrow_forward
- (a) Draw the structure of the following :(i) p-Methylbenzaldehyde (ii) 4-Methylpent-3-en-2-one(b) Give chemical tests to distinguish between the following pairs of compounds :(i) Benzoic acid and Ethyl benzoate, (ii) Benzaldehyde and Acetophenone.(iii) Phenol and Benzoic acid.arrow_forwardDimethyl disulfide, CH,S–SCH3, found in the vaginal secretions of female hamsters, acts as a sexual attractant for the male hamster. Write an equation for its synthesis from methanethiol.arrow_forward(a) Tsomane and Nyiko were given a task of synthesising methylenecyclohexane 2. After a brief discussion with each other, Tsomane proposed Method A to synthesise 2 from cyclohexanone 1 while Nyiko proposed Method B that started from hydroxymethylcyclohexane 3. Each student believed that their proposed method is better than the other. (Scheme below) (1) Ph Ph 8*8 Ph THF A 1 Santande B H₂SO4 100 °C 3 OH Using curly arrows, provide full mechanistic details accounting how methylenecyclohexane 2 was synthesised according to both Methods A and B.arrow_forward
- Develop syntheses for the following compounds, using acetylene and compounds containing no more than four carbon atoms as your organic starting materials.(a) 3-methylnon-4-yn-3-ol (“3-ol” means there is an OH group on C3.)(b) cis-1-ethyl-2-methylcyclopropanearrow_forwardA postgraduate student wanted to synthesized two carbonyl compounds known as compounds M and N using oxidation of alcohol. However, these carbonyl compounds should have more than 6 carbon atoms. Draw two (2) possible structural formulae for compounds M and N. How would you distinguish between compounds M and N?arrow_forward(a) Based on what you know about the relative stabilities of alkyl cations and benzylic cations, predict the product of addition of HBr to 1-phenylpropene.(b) Propose a mechanism for this reaction.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





