What is IR Spectrum of Cyclohexanone?
It is the analysis of the structure of cyclohexaone using IR data interpretation.
Cyclohexanone
Cyclohexanone is a cyclic molecule consisting of six carbon molecules that has a functional group consisting of ketone with the molecular formula of C6H10O. It is a fluid, whose smell resembles acetone, and is colorless.
Characteristics of Cyclohexanone
Cyclohexanone is water-soluble and miscible in almost all organic solvents.
The flash point of cyclohexanone is 46°C and has a specific gravity of 0.945. It is mildly water soluble and highly soluble with common solvents. It naturally occurs in crude oils and has been synthetically processed in significant amounts as it is a major intermediary for nylon manufacturing. Cyclohexanone synthesis is dependent upon cyclohexane oxidation or on the benzene hydrogenation, in air and mostly in the existence of crystals of cobalt.
Cyclohexanone use is attributed to the nylon sector nearly exclusively through related products that are the accompanying nylon-6 such as adipic acid as well as caprolactam. For manufacturing drugs, dyes, herbicides, poisons, plasticizers, even rubber compounds, several cyclohexanone variants are being used.

Properties of Cyclohexanone
Cyclohexanone shows following properties:
- Oily colorless or light yellow in color.
- Water soluble as well as in solvents such as alcohol and ether.
- Typical aroma similar to acetone.
- It has a molecular weight of 98.14 .
- It has a density of 0.9478 g/cm 3.
- It has a melting point of −31 °C.
- It has a boiling point of 155.6° C.
Infrared Absorption of Cyclo-Hydrocarbons
The hydrocarbon’s infrared absorbance value is determined using characteristic bands to identify it. Such data is also useful for measuring product quality. Some research was conducted in small spectrum areas, such as 1 to 2 µ, to find out if the variation in spectrum between different forms of hydrocarbons was necessary to identify the effects. The number of bands detected at 3.4 µ may exceed two in cyclohexane, which has far more than two CH2 groups because of group associations. The relation between the CH2 group as well as the molecule will alter the band's position. The size of the rest of the sample thus has no impact on the location of the two different bands. In addition, in the area of 3.4 µ, compounds that are not hydrocarbons, however include CH2 groups. The formaldehyde compound does have relatively small rates of wave vibration than any of those discovered in hydrocarbon for CH2 groups, but still the rates of OHCH2 seem to be greater in fluoreneCl3HIO.
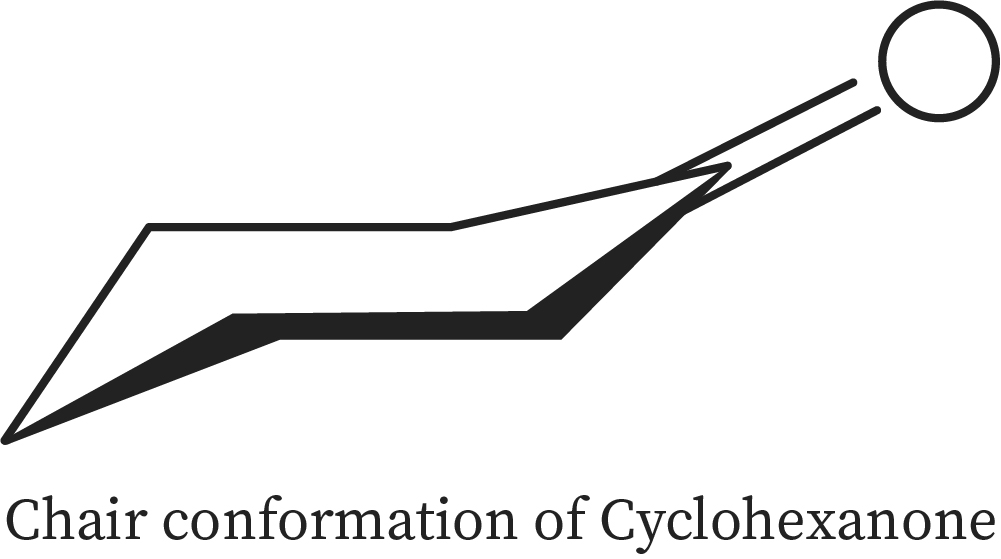
Cyclohexanone Conformations
Cyclohexanone is usually observed in two arrangements; they are chair form and boat form. Cyclohexanone is found to be in chair form when observed in gaseous state. Cyclohexanone also shows two of the boat and two of the chair forms where the chair arrangement is found to be extra stable when compared to the boat form.
Infrared Spectroscopy
Infrared (IR) spectroscopy is the most commonly used methods for spectroscopy. In a specific section of the wavelength in the infrared region absorbing groups show absorption. In this area, the absorption peaks are normally stronger than those of the ultraviolet – visible zones. This allows IR spectroscopy to be highly sensitive to functional groups being determined inside the sample, as separate functional classes absorb various IR specific frequencies. Furthermore, each substance has a typical signature range. Through matching its absorption peak with a spectrum database, one molecule may be characterized. The frequency of the IR radiation is lower than that of the intensity of the visible range and also of the ultraviolet radiation. IR absorption is common for molecular species with a low energy gap among rotational and vibratory states. The net shift of dipole moment in a substance when it vibrates or revolves is a prerequisite for IR absorption.
Infrared spectroscopy applications
- It helps in identification of the different functional groups present in any given sample.
- It also helps in identifying any impurity present in the given sample by showing additional peaks in the IR spectrum.
- It also gives us a distinction on hydrogen bonding whether it is intermolecular or else intramolecular.
- It also helps in identification of cis and trans isomers of a given compound.
Ir absorption spectra
Following are the absorption spectra for few compounds
| Types of vibration | Frequency (cm-1) | |
| Alkane | C-C stretching | 1200 |
| Alkenes | C=H stretching | 1650 |
| Alkynes | C≡C stretching | 2100 |
| C=O stretching | Amide | 1680 |
| C=O stretching | Ketone | 1715 |
| O-H Alcohol, phenol | Free | 3600 |
Infrared absorption of ketones
Aliphatic saturated ketones show a carbonyl stretching vibration at around 1715 cm−1.Any conjugation of these carbonyl functional groups with double bonds between two adjacent carbon atoms or with phenyl group as observed in unsaturated aldehydes as well as benzaldehyde will shift this peak lower to around 1685-1666 cm−1.
Infrared spectra of cyclohexanone
Cyclohexanone is a cyclic hydrocarbon molecule that has a ketone acting as its functional group. So, the infrared spectrum of cyclohexanone shows sharp peaks at definite wave number corresponding to its functional groups present. Since cyclohexanone is a saturated hydrocarbon without any unsaturated double or else triple bonds all the bonds present are of sp3 hybridization. This can be observed in the IR spectra of cyclohexanone where a sharp peak is observed around 1715 cm−1confirming the existence of a ketone group. The graph also shows other peaks but the main stretch that determines its functional group can be seen at 1715 cm−1, whereas, there is no sharp peak at 1685-1666 cm−1indicating no conjugation with any other groups. The graph also shows absence of any sharp peaks around 1150 -1075 cm−1 indicating absence of alcohols present hence helping us differentiate between a cyclohexanone from a cyclohexanol.
Common Mistakes
Students must be able to identify the peaks while solving questions. Any wrong peak identification may result in identifying a wrong compound.
Context and Applications
This topic is significant in the professional exams for both undergraduate and graduate courses, especially for Bachelors and Masters in Chemistry.
Want more help with your chemistry homework?
*Response times may vary by subject and question complexity. Median response time is 34 minutes for paid subscribers and may be longer for promotional offers.
IR Spectrum Of Cyclohexanone Homework Questions from Fellow Students
Browse our recently answered IR Spectrum Of Cyclohexanone homework questions.