
(a)
Interpretation: The product of NADH reduction of ethanal in presence of alcohol dehydrogenase should be written.
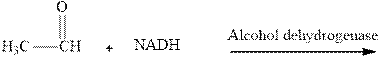
Concept introduction:NADH is cofactor essential for
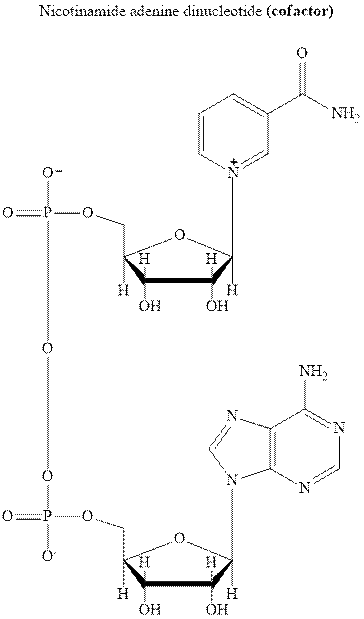
It is the biochemical redox reagent that adds hydride ion for reduction of carbonyl compounds except for the carbonyl moiety of
(b)
Interpretation: The product of NADH reduction of pyruvic acid in presence of lactate dehydrogenase should be written.
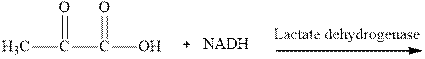
Concept introduction: NADH is the cofactor essential for metabolic processes. It comprises of two nucleotides linked via phosphate groups as illustrated below.
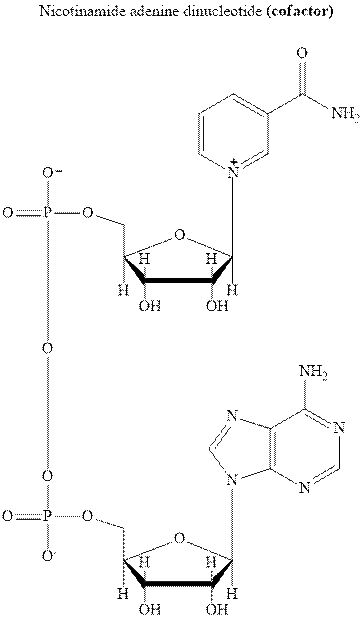
It is the biochemical redox reagent that add hydride ion for reduction of carbonyl compounds except the carbonyl moiety of carboxylic acids.
(c)
Interpretation: The product of NADH reduction of oxaloacetic acid in presence of malate dehydrogenase should be written.
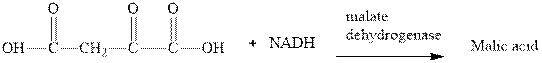
Concept introduction: NADH is the cofactor essential for metabolic processes. It comprises of two nucleotides linked via phosphate groups as illustrated below.
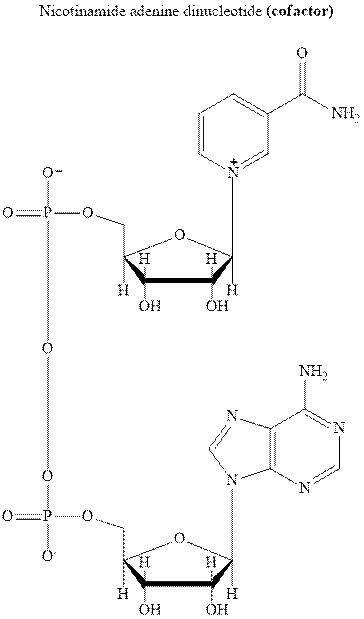
It is the biochemical redox reagent that add hydride ion for reduction of carbonyl compounds except the carbonyl moiety of carboxylic acids.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry: Structure and Function
- In a time-resolved picosecond spectroscopy experiment, Sheps, Crowther, Carrier, and Crim (Journal of Physical Chemistry A, Vol. 110, 2006; pp. 30873092) generated chlorine atoms in the presence of pentane. The pentane was dissolved in dichloromethane, CH2C12. The chlorine atoms are free radicals and are very reactive. After a nanosecond the chlorine atoms have reacted with pentane molecules, removing a hydrogen atom to form HCl and leaving behind a pentane radical with a single unpaired electron. The equation is Cl (dcm) + C5H12(dcm) HCl(dcm) + C5H11 (dcm) where (dcm) indicates that a substance is dissolved in dichloromethane. Measurements of the concentration of chlorine atoms were made as a function of time at three different concentrations of pentane in the dichloromethane. These results are shown in the table. (a) Determine the order of the reaction with respect to chlorine. (b) Determine whether the reaction rate depends on the concentration of pentane in dichloromethane. If so, determine the order of the reaction with respect to pentane. (c) Explain why the concentration of pentane in dichloromethane does not affect the data analysis that you performed in part (a). (d) Write the rate law for the reaction and calculate the rate of reaction for a concentration of chlorine atoms equal to 1.0 M and a pentane concentration of 0.23 M. (e) Sheps, Crowther, Carrier, and Crim found that the rate of formation of HCl matched the rate of disappearance of Cl. From this they concluded that there were no intermediates and side reactions were not important. Explain the basis for this conclusion.arrow_forwardWrite an equation for the reaction of chloroacetic acid (Ka=1.5103) with trimethylamine (Kb=5.9105) . Calculate the equilibrium constant for the reaction. If 0.10 M solutions of these two species are mixed, what will be their concentrations at equilibrium?arrow_forwardAll amino acids share a common structure, a central (alpha) carbon with four groups attached. Which of these is NOT one of the four groups attached to the alpha- carbon? O an R group an ammonium group (-NH3+) O a carboxylate group (-COO) a hydronium grouparrow_forward
- Amino acids containing a nonpolar "R" group, such as an alkyl or aromatic, are in nature. acidic O hydrophobic O hydrophilic O basicarrow_forwardIs the alpha hydrogen of an ester such as RCH2COOC2H5 acidic? Explain in words.arrow_forwardIs the alpha hydrogen of an ester such as RCH2COOC2H5 acidic? Explain.arrow_forward
- PAP Chemistry-2903012-42100P-1/ Le Chatelier's Principle/ Lesson 128 2. Zinc (Zn) granules react slowly with dilute hydrochloric acid (HCI), but much faster if the acid is concentrated. Zn(s) 2HCI(aq)ZnCl2(aq) + H2(g) Zinc + Hydrochloric Acid Zinc Chloride + Hydrogen What causes the reaction to proceed faster with concentrated acid? The concentrated hydrochloric acid causes more hydrogen gas to be produced. The pressure of hydrogen gas molecules increases as concentration increases. The concentrated hydrochloric acid molecules move faster than in dilute acid. There are more collisions between the zinc and concentrated hydrochloric acid. PREVIOUarrow_forwardMethanogens are prokaryotes that produce methane according to the net equation H2 + CO2→CH4 + 2H2O. Some bacteria consume methane according to the net equation CH4 + SO42- →HCO3- + HS− + H2O. (a) Classify these two types of bacteria as autotrophic or heterotrophic. (b) Explain why the two types of bacteria are often found associated with each other.arrow_forwardAnother source of phosphorus in water is known as organic phosphorus which commonly finds its way into natural bodies of water from human and animal waste and food residues. If you wish to measure the phosphorus content due to organic matter in the water sample using the vanadomolybdophosphoric acid method, you must first "digest" the sample to convert the organic phosphorus to orthophosphate. O True O Falsearrow_forward
- 21. Two fatty acids found in rapeseed oil are behenic acid and erucic acid. Both contain the same number (22) of carbon atoms. Behenic acid is saturated and erucic acid is monounsaturated. The abbreviated structural formula of erucic acid is CH, (CH,),CH=CH-(CH2)11COOH. (a) Deduce the abbreviated structural formula for behenic acid 4 b) Explain why the melting point of erucic acid is lower than that of behenic acid 22. A cell membrane can vary in terms of how "fluid" or "flexible" it is. Describe if you would expect each of the following changes to make a particular membrane more fluid or more rigid. Explain your choices. a) An increase in the cholesterol content b) An increase in the fraction of unsaturated fatty acyl tails in the membrane phospholipids.arrow_forwardWrite the products of the following sequence of reactions.arrow_forwardConsider the following amino acid with an isoelectric point pH 6.06 O H NH₂ OO The structure of this amino acid is in its The name of the amino acid is formarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


