
Concept explainers
Interpretation:The reason behind excess usage of methyl magnesium iodide andmethyl lithium and number of equivalents of methyl magnesium iodide and methyl lithiumneeded in each case along with the products formed at functionalized sites should be given.
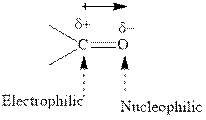
Concept introduction: The carbonyl bond is polar with partial positive charge on carbon and partial negative charge on oxygen as illustrated below.
Grignard reagents are
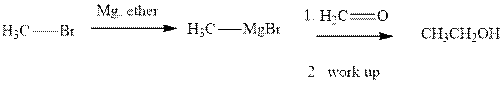
Methyl bromide reacts with magnesium ether to give Grignard reagent. This Grignard methyl magnesium bromide on treatment with formaldehyde gives corresponding alcohol. The mechanistic pathway for the latter reaction is as follows:
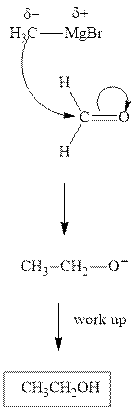
The polar carbonyl bond breaks and the polar Grignard reagent attack at electron-deficient carbon. Finally with the hydrolysis and work up the alcohol is formed.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry: Structure and Function
- 2. The following reactions do not work well to produce the given substitution product. Explain why. OH Br Br 1. NaH 2. KSCH3 KCN H₂O SCH3 CN OHarrow_forwardThe Zeisel method is an old analytical procedure for determining the number of methoxyl groups in a compound. A weighed amount of the compound is heated with concentrated HI, ether cleavage occurs, and the iodomethane product is distilled off and passed into an alcohol solution of AgNO3, where it reacts to form a precipitate of silver iodide. The Agl is then collected and weighed, and the percentage of methoxyl groups in the sample is thereby determined. For example, 1.06 g of vanillin, the material responsible for the characteristic odor of vanilla, yields 1.60 g of Agl. If vanillin has a molecular weight of 152, how many methoxyl groups does it contain?arrow_forwardThe reaction in the box is missing a reagent/reactant... NCH3 NHCH3 Which of the following would you use to convert the starting material into the product in high yield? A) An oxidation reagent such as H2CrO4 B) A base such as NaOH/H20 C) An acid such as H2SO4 D) A reduction agent such as NABH4 A В C O Darrow_forward
- When 3,4-dimethyl-2,4-hexadiene reacts with one equivalent of HBr, two products are formed. a. Draw the 1,2-addition product and the mechanism for its formation. ۱۸ HBr Br Br b. Draw the 1,4-addition product and the mechanism for its formation. M H-Br یامده بله ا پاره OP ONLarrow_forward2. Draw the products for the following reaction conditions using the starting material provided. 1. CH3LI; 2. H2O 1. CH3CH2MgBr 2.H20 1.(CH2CH)2CuLi 2. H20arrow_forward5. Draw the major product of the following reactions. Classify each reaction as substitution, elimination, or addition. Indicate if there are any rearrangement products. If the reaction does not proceed, explain why. 1) BH3 THF 2) HOOH, OH CI NaN3 (1 equiv) THFarrow_forward
- 36. Which of the following is the major E2 product formed from the following alkyl halide? OI ||| O IV Both I and II II CI III IVarrow_forwardQUESTION 33 Which of the following rate laws accurately describes the rate determining step in an Et elimination of alcohols? OA Rate - Mearbocationproton OH. Rate = kfoxonium lon)[alcohol O cRate kfcarbocation] OD Rate = loxonium lon] OE Rate kcarbocation][water) QUESTION 34 Aqtuieous KMnO4 which is purple, indicatos the presence of alkenos by reacting with them to forn coioriess precipitate and OA. none of the answers OR 12 diols, molybdenum dioxide, orange Or vicinal diols, manganese dioxide. brown O D. glycols, magnesium dioxide, brown OE alkyl chlorides, potasslum hydroxide, yellow O F. geminal diols, manganese dioxide, brown QUESTION 35 five carbon (S)-(+) Carvone was isolated from caraway seeds and belongs to a group of natural compounds called O steralds, one pentane O alkaloids, three, tert-butyl O terpenoids, two, isoprene which contain subunit(s) pentanoids, one, pentane O None of the abovearrow_forward#16d. Provide the missing reactants, reagents, or products for the following reaction sequences below.arrow_forward
- Complete the reaction by providing the starting material/reagent/products. Match each item to a choice: HO OH NO₂ Cl₂ FeCl3 HO SO3 conc. H₂SO4 KMnO4 H₂O NO₂ NO₂ CIarrow_forwardQV:Using only the reagents from this list, show the synthesis of this compound Reagents that can be used (you can use the reagent more than one time, if needed) Br HO- Br NaH Heat CH,OH Br2 CH,COOH HCI H2SO, Cro, Pyridinearrow_forward↑ Draw the major product of this reaction. Ignore inorganic byproducts. 68°F Wind OH oi Na2Cr2O7 H₂SO4, H₂O Oarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

