
Interpretation:The direction of equilibrium that is favoredshould be identified.
Concept introduction:In accordance with Bronsted definition an acid can act as a proton donor and a base can act as a proton acceptor. Thus in a typical acid-base reaction, the fundamental principle is a lone pair of base reaches out for an acidic proton. Similar curved arrows are used to show the movement of electrons. After deprotonation, the species left with a negative charge is referred as the conjugate base of acid while the other with a positive charge is termed conjugate acid of given base. For example,
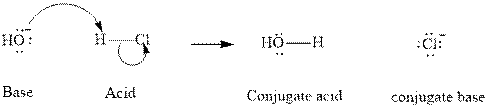
The strength of various conjugate acid-base pairs varies inversely to one another; the strong acid has a weak conjugate base and the strong base has weak conjugate acid and vice-versa.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry: Structure and Function
- Will acetylene react with sodium hydride according to the following equation to form a salt and hydrogen, H2? Using pKa values given in Table 4.1, calculate Keq for this equilibrium.arrow_forwardIf the G for a reaction is 4.5 kcal/mol at 298 K, what is the Keq for this reaction? What is the change in entropy of this reaction if H = 3.2 kcal/mol?arrow_forwardComplete the following acid-base reactions. (a) CH3CH2CH2CH2Li + CH3COOH (b) CH3CH2CH2CH2MgBr + CH3CH2OHarrow_forward
- The Keq for the reaction: A + B ↔ AB is 4.32 What is the Keq for 2 A + 2 B ↔ 2 AB Keq = ?arrow_forwardWhat will be the product for the following reaction? H + HO OH -CO₂arrow_forwardAcetic acid, CH3COOH, is a weak organic acid, pKa 4.76. Write an equation for the equilibrium reaction of acetic acid with each base. Which equilibria lie considerably toward the left? Which lie considerably toward the right? Q.) NaHCO3arrow_forward
- The following reaction is reversible under acidic conditions: + H₂O f What step can be done to shift the equilibrium towards formation of more products? O Use excess amount of the alcohol O Use excess amount of the carboxylic acid O Use excess amount of water O Continuously remove water from the mixture O All of these O OH + CH3OHarrow_forwardWhich of the following species is the strongest acid in an aqueous solution? CH3CH2CO2H CH2ClCO2H Ch3CO2H CCl3CO2H CHCl2CO2Harrow_forwardWhich is the strongest acid? O CH3NH₂ O CH3OHarrow_forward
- Which of the following reactions are correct? Explain the answer. + H₂O + H₂O H₂SO4 H OH H OHarrow_forwardGive the products of the föllówing twó áčid-bášé réáčtións ánd for eách indicate whether reactants or products are favored at equilibrium. CHCH,ОH + CH;NH3* CH;CH,OH CH;NHNAarrow_forwardWhich of the following molecules is the strongest base? CH3 CH3 OH CH3 CH3arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
