
Organic Chemistry: Structure and Function
8th Edition
ISBN: 9781319079451
Author: K. Peter C. Vollhardt, Neil E. Schore
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 8, Problem 49P
Interpretation Introduction
Interpretation:The reason Wurtz coupling method as not a useful synthetic method for two different alkyl groups should be suggested.
Concept introduction:Organometallic compounds are highly reactive towards haloalkanes in presence of electropositive metal. Wurtz coupling involves the two equivalents of
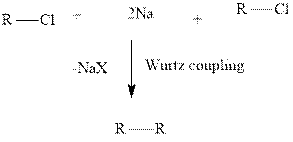
Limitation of this coupling method is that it can only be used in synthesis of symmetrical
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Alkynes do not react directly with aqueous acid as do alkenes, but will do so in the presence of mercury(II) sulfate as a Lewis acid catalyst. The reaction occurs with Markovnikov
regiochemistry, so the OH group adds to the more highly substituted carbon and the H adds to the less highly substituted carbon. The initial product of the reaction is a vinyl alcohol, also called
an enol. The enol immediately rearranges to a more stable ketone via tautomerization.
Draw curved arrows to show the movement of electrons in this step of the mechanism.
Arrow-pushing Instructions
-X티
Hö:
H-O
-CH3
-CH3
H30*
QUESTION 7
Indicate whether the following species are electrophile or nucleophile
i)
ii)
iii)
H20
Cl2
NO2"
+
iv)
CH2=CH2
As we have seen in this chapter, carbon-carbon double bonds are electron-rich regions
that are attacked by electrophiles (e.g., HBr); they are not attacked by nucleophiles
(e.g, diethylamine).
electrophilic
addition
HBr +
Br
(a racemic mixture)
Et,NH +
No reaction
Diethylamine
(a nucleophile)
However, when the carbon-carbon double bond has a carbonyl group adjacent to it, the
double bond reacts readily with nucleophiles by nucleophilic addition
Chapter 8 Solutions
Organic Chemistry: Structure and Function
Ch. 8.1 - Prob. 8.1ECh. 8.1 - Prob. 8.2ECh. 8.3 - Prob. 8.4TIYCh. 8.3 - Prob. 8.5ECh. 8.3 - Prob. 8.6ECh. 8.4 - Prob. 8.7ECh. 8.5 - Prob. 8.8ECh. 8.5 - Prob. 8.9ECh. 8.5 - Prob. 8.10ECh. 8.5 - Prob. 8.11E
Ch. 8.5 - Prob. 8.12ECh. 8.6 - Prob. 8.14TIYCh. 8.7 - Prob. 8.15ECh. 8.7 - Prob. 8.16ECh. 8.8 - Prob. 8.18TIYCh. 8.8 - Prob. 8.19ECh. 8.8 - Prob. 8.21TIYCh. 8 - Prob. 24PCh. 8 - Prob. 25PCh. 8 - Prob. 26PCh. 8 - Prob. 27PCh. 8 - Prob. 28PCh. 8 - Prob. 29PCh. 8 - Prob. 30PCh. 8 - Prob. 31PCh. 8 - Prob. 32PCh. 8 - Prob. 33PCh. 8 - Prob. 34PCh. 8 - Prob. 35PCh. 8 - Prob. 36PCh. 8 - Prob. 37PCh. 8 - Prob. 38PCh. 8 - Prob. 39PCh. 8 - Prob. 40PCh. 8 - Prob. 41PCh. 8 - Prob. 42PCh. 8 - Prob. 43PCh. 8 - Prob. 44PCh. 8 - Prob. 45PCh. 8 - Prob. 46PCh. 8 - Prob. 47PCh. 8 - Prob. 48PCh. 8 - Prob. 49PCh. 8 - Prob. 50PCh. 8 - Prob. 51PCh. 8 - Prob. 52PCh. 8 - Prob. 53PCh. 8 - Prob. 54PCh. 8 - Prob. 55PCh. 8 - Prob. 56PCh. 8 - Prob. 57PCh. 8 - Prob. 58PCh. 8 - Prob. 59PCh. 8 - Prob. 60PCh. 8 - Prob. 61PCh. 8 - Prob. 62PCh. 8 - Prob. 63PCh. 8 - Prob. 64PCh. 8 - Prob. 65PCh. 8 - Prob. 66P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the product of the two reactions shown below. In the first step indicate if the product is meso or is formed as a racemic mixture. Indicate whether final structure in the second reaction is cis or trans. Ph Br-Br H. LDA, THF solvent |arrow_forwardwhat is the best way to come up with how to synthesize carboxylic acid , oct-4-yne to butanoic acid, or trans cyclodecene to decanedoic acid . I keep getting confused on how to go from the reactants to product, even with the amines. i can't figure out what to use to get to the product. Please explain Grignard rx as well.arrow_forwardWhat are the steps of the mechanism of reaction . Electrophilic aromatic substitution. Is it Ortho,para,meta ?arrow_forward
- What would be the major product obtained from the reaction of Br2 with 1-butene if the reaction were carried out in the solvent dichloromethane? Draw the molecule.arrow_forwardWhich of the following is not an electrophile * AIB13 BF3 SnCl4 NH3arrow_forwardNomenclature: provide the systematic names or structure of each of the following compounds. Include E/Z/R/S where relevant.arrow_forward
- When 2-methylpropane is monochlorinated in the presence of light at room temperature, 36% of the product is 2-chloro-2-methylpropane and 64% is 1-chloro-2-methylpropane. From these data, calculate how much easier it is to remove a hydrogen atom from a tertiary carbon than from a primary carbon under these conditions.arrow_forwardDraw resonance forms to show how the BHA radical is stabilized by delocalization of the radical electron over other atoms in the molecule.arrow_forwardDraw the structure of the major product of following reactions: CH2l2, Zn(Cu) Et,0 „MgBr H3O* to H,0 2. Zn 3. ..... HCI CH212, Zn(Cu) 4. Et,0 H3O* + CH;CH2M9B. 5,arrow_forward
- The following reaction is electrophilic substitution: 3- [Pt(CH₂) (N) [Pt(CH3)(CI) (PMePh₂)₂] + N° (PMePh₂)₂] + CI Select one: O True O Falsearrow_forwardWhat is Product B? ta CI Br2 FeBr3 AIC13 Br Br Brarrow_forwardWhich of the following assertions most accurately defines alkynes' overall reactivity? Alkynes undergo electrophilic addition reactions just like alkenes. An alkyne reacts as an electrophile and is therefore electron rich. An alkyne reacts as a nucleophile and is therefore electron poor. An alkyne reacts as an electrophile and is therefore electron poor.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License