
Concept explainers
(a)
Interpretation: Synthesis of
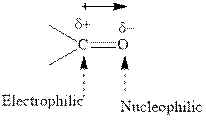
Concept introduction: The carbonyl bond is polar with the partial positive charge on carbon and partial negative charge on oxygen as illustrated below.
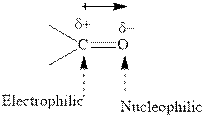
Grignard reagents are
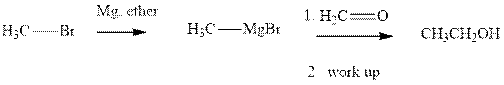
Methyl bromide reacts with magnesium ether to give Grignard reagent. This Grignard methyl magnesium bromide on treatment with formaldehyde gives corresponding alcohol. The mechanistic pathway for the latter reaction is as follows:

The polar carbonyl bond breaks and the polar Grignard reagent attack at electron-deficient carbon. Finally with the hydrolysis and work up the alcohol is formed.
(b)
Interpretation: Synthesis of
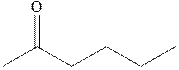
Concept introduction: The carbonyl bond is polar with the partial positive charge on carbon and partial negative charge on oxygen as illustrated below.
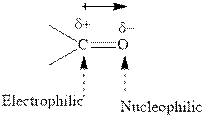
Grignard reagents are alkyl magnesium halides obtained from treatment of haloalkane with magnesium in presence of dried ether conditions. These reagents are useful precursors for quick synthesis of variety of organic compounds For example, the reaction of Grignard reagent with aldehyde or ketone generates alcohol as illustrated below.
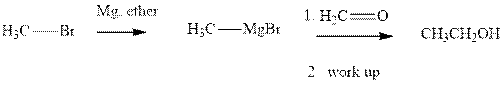
(c)
Interpretation: Synthesis of
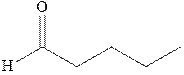
Concept introduction: The carbonyl bond is polar with the partial positive charge on carbon and partial negative charge on oxygen as illustrated below.
Grignard reagents are alkyl magnesium halides obtained from treatment of haloalkane with magnesium in presence of dried ether conditions. These reagents are useful precursors for quick synthesis of variety of organic compounds For example, the reaction of Grignard reagent with aldehyde or ketone generates alcohol as illustrated below.
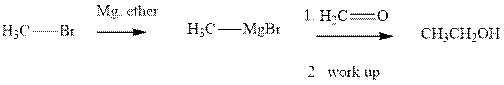
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry: Structure and Function
- provide appropriate reagentsarrow_forwardShow how to synthesize the following product as the major product starting with 2,2-dimethylpropane as the starting material. You may use additional reagents and any number of steps. Be sure to list each step with all reactants/reagents/conditions required. (Do not use hydrogenation reactions). Write the process.arrow_forwardSynthesize each compound from benzene. Use a diazonium salt as one of the synthetic intermediates.arrow_forward
- A synthetic organic molecule, G, which contains both aldehyde and ether functional groups, is subjected to a series of reactions in a multi-step synthesis pathway. In the first step, G undergoes a Wittig reaction, leading to the formation of an alkene, H. Subsequently, H is treated with an ozone (O3) reagent followed by a reducing agent in an ozonolysis reaction, resulting in the formation of two different products, I and J. Considering the functional groups present in G and the nature of the reactions involved, what are the most probable structures or functional groups present in products I and J? A. I contains a carboxylic acid group, and J contains an aldehyde group. B. I contains a ketone group, and J contains an alcohol group. C. I and J both contain aldehyde groups. D. I contains an ester group, and J contains a ketone group. Don't use chat gpt.arrow_forwardSynthesize 2-pentanone from 1-butyne. Indicate all the reagents needed.arrow_forwardShow how to synthesize the following product as the major product starting with 2,2- dimethylpropane as the starting material. You may use additional reagents and any number of steps. Be sure to list each step with all reactants/reagents/conditions required. (Do not use hydrogenation reactions) fromarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





