
Concept explainers
(a)
Interpretation: The indicated compound should be named.
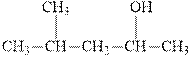
Concept introduction: In accordance with
As per IUPAC recommendations, longest chain found in a continuous manner in a branched molecule is chosen as parent chain. The longest chain must contain the alcohol
The chain carbons are numbered so as to provide least numeral position towards the end in vicinity to
For
(b)
Interpretation:The indicated compound should be named.
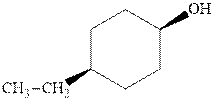
Concept introduction:In accordance with IUPAC systematic nomenclature od alcohols can be derived from the parent alkane with “e” dropped and replaced by “ol” suffix.
As per IUPAC recommendations, longest chain found in a continuous manner in a branched molecule is chosen as parent chain. The longest chain must contain the alcohol functional group.
The chain carbons are numbered so as to provide least numeral position towards the end in vicinity to
For alkanes side chains the positions of side chains or alkyl substituents are indicated by lowest numeral places before prefix.
(c)
Interpretation: The indicated compound should be named.
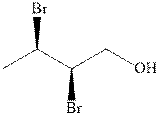
Concept introduction:In accordance with IUPAC systematic nomenclature od alcohols can be derived from the parent alkane with “e” dropped and replaced by “ol” suffix.
As per IUPAC recommendations, longest chain found in a continuous manner in a branched molecule is chosen as parent chain. The longest chain must contain the alcohol functional group.
The chain carbons are numbered so as to provide least numeral position towards the end in vicinity to
For alkanes side chains the positions of side chains or alkyl substituents are indicated by lowest numeral places before prefix.
In order to assign absolute configuration of R and S, Cahn − Ingold − Prelog rules are used and the first step is to assign the priority order on the basis of
The Fischer projection is written along with the priorities assigned and groups are interchanged between adjacent places so as to obtain lowest priority group at the bottom or lowest priority.
If the groups now are arranged are read from highest towards least in clockwise fashion the R is assigned to the stereocenter, if the rotation is anticlockwise the S is assigned at the configuration.
(d)
Interpretation: The indicated compound should be named.
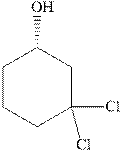
Concept introduction:In accordance with IUPAC systematic nomenclature od alcohols can be derived from the parent alkane with “e” dropped and replaced by “ol” suffix.
As per IUPAC recommendations, longest chain found in a continuous manner in a branched molecule is chosen as parent chain. The longest chain must contain the alcohol functional group.
The chain carbons are numbered so as to provide least numeral position towards the end in vicinity to
For alkanes side chains the positions of side chains or alkyl substituents are indicated by lowest numeral places before prefix.
In order to assign absolute configuration of R and S, Cahn − Ingold − Prelog rules are used and the first step is to assign the priority order on the basis of atomic number. The one with highest atomic number gest highest priority and is designated as a and so on.
The Fischer projection is written along with the priorities assigned and groups are interchanged between adjacent places so as to obtain lowest priority group at the bottom or lowest priority.
If the groups now are arranged are read from highest towards least in clockwise fashion the R is assigned to the stereocenter, if the rotation is anticlockwise the S is assigned at the configuration.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry: Structure and Function
- Name the following molecule by following IUPAC rules. Use appropriate stereochemical designations (R, S, E, or Z) as needed. Do not put spaces between dashes or commas in the answer.arrow_forward[Review Topics] [References] Use the References to access important values if needed for this question. Draw the structural formula for the intermediate species that forms when the following compound is treated with K₂Cr₂O7. CH₂CH3 CH₂CH₂CHCH₂OH T • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. ▾ K₂Cr₂O7 H₂SO4 will CH₂ ? • O-Sn [F ? Harrow_forwardDraw the major organic product(s) of the following reactions including stereochemistry when it is appropriate. H20/ H,SO, / HgSO4 CH,CH2-CEC-CH, • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If no reaction occurs, draw the organic starting material. Separate multiple products using the + sign from the drop-down menu.arrow_forward
- Write the IUPAC name of the following molecule: "Lowercase letters only and DO NOT put space in between. Gray balls represent C, white balls represent H, green ball represent F. Do not include stereochemistry. * Your answerarrow_forwardThese are synthesis questions. You need to show how the starting material can be converted into the product(s) shown. You may use any reactions we have learned. Show all the reagents you need. Show each molecule synthesized along the way and be sure to pay attention to the regiochemistry and stereochemistry preferences for each reaction. If a racemic molecule is made along the way, you need to draw both enantiomers and label the mixture as "racemic". All of the carbon atoms of the products must come from the starting material(s)! LOH ? H E,Z Mixture Harrow_forwardThese are synthesis questions. You need to show how the starting material can be converted into the product(s) shown. You may use any reactions we have learned. Show all the reagents you need. Show each molecule synthesized along the way and be sure to pay attention to the regiochemistry and stereochemistry preferences for each reaction. If a racemic molecule is made along the way, you need to draw both enantiomers and label the mixture as "racemic". All of the carbon atoms of the products must come from the starting material(s)! H ? Racemic Harrow_forward
- These are synthesis questions. You need to show how the starting material can be converted into the product( s) shown. You may use any reactions we have learned. Show all the reagents you need. Show each molecule synthesized along the way and be sure to pay attention to the regiochemistry and stereochemistry preferences for each reaction. If a racemic molecule is made along the way, you need to draw both enantiomers and label the mixture as "racemic". ? OH carrow_forwardThese are synthesis questions. You need to show how the starting material can be converted into the product(s) shown. You may use any reactions we have learned. Show all the reagents you need. Show each molecule synthesized along the way and be sure to pay attention to the regiochemistry and stereochemistry preferences for each reaction. If a racemic molecule is made along the way, you need to draw both enantiomers and label the mixture as "racemic". All of the carbon atoms of the products must come from the starting material(s)! OH ?arrow_forwardThese are synthesis questions. You need to show how the starting material can be converted into the product(s) shown. You may use any reactions we have learned. Show all the reagents you need. Show each molecule synthesized along the way and be sure to pay attention to the regiochemistry and stereochemistry preferences for each reaction. If a racemic molecule is made along the way, you need to draw both enantiomers and label the mixture as "racemic". All of the carbon atoms of the products must come from the starting material(s)! ? suz يين (racemic)arrow_forward
- These are synthesis questions. You need to show how the starting material can be converted into the product(s) shown. You may use any reactions we have learned. Show all the reagents you need. Show each molecule synthesized along the way and be sure to pay attention to the regiochemistry and stereochemistry preferences for each reaction. If a racemic molecule is made along the way, you need to draw both enantiomers and label the mixture as "racemic". All of the carbon atoms of the products must come from the starting material(s)! bl H ? O: Racemicarrow_forwardThese are synthesis questions. You need to show how the starting material can be converted into the product(s) shown. You may use any reactions we have learned. Show all the reagents you need. Show each molecule synthesized along the way and be sure to pay attention to the regiochemistry and stereochemistry preferences for each reaction. If a racemic molecule is made along the way, you need to draw both enantiomers and label the mixture as "racemic". All of the carbon atoms of the products must come from the starting material(s)! ? عنیarrow_forwardWhat is the name of this molecule? Don't forget to include stereochemistry! Br OHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





