
(a)
Interpretation: The mechanism for formation of product in indicated reaction should be written.
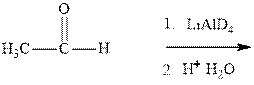
Concept introduction:The carbonyl bond is polar with partial positive charge on carbon and partial negative charge on oxygen as illustrated below.
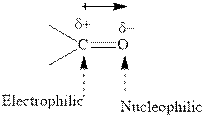
Thus it can undergo hydride addition at carbon and proton addition at oxygen. Certain reagents that are useful for such hydride addition at carbonyl carbon include
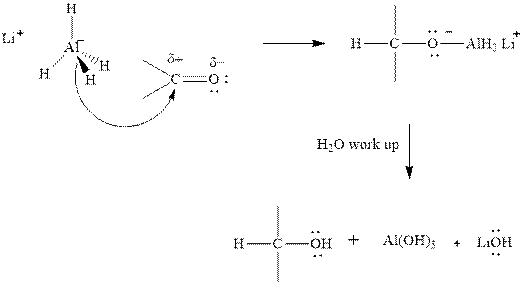
(b)
Interpretation:The mechanism for formation of product in indicated reaction should be written.

Concept introduction:The carbonyl bond is polar with partial positive charge on carbon and partial negative charge on oxygen as illustrated below.
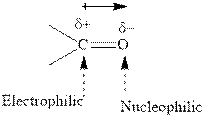
Thus it can undergo hydride addition at carbon and proton addition at oxygen. Certain reagents that are useful for such hydride addition at carbonyl carbon include
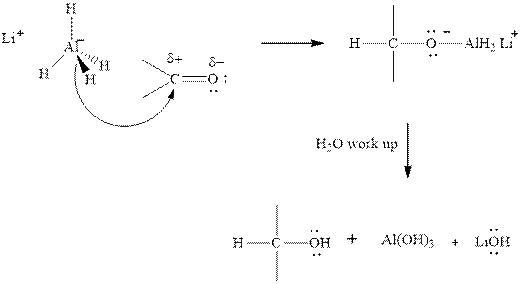
(c)
Interpretation:The mechanism for formation of product in indicated reaction should be written.
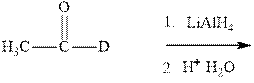
Concept introduction:The carbonyl bond is polar with partial positive charge on carbon and partial negative charge on oxygen as illustrated below.
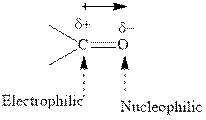
Thus it can undergo hydride addition at carbon and proton addition at oxygen. Certain regents that are useful for such hydride addition at carbonyl carbon include
(d)
Interpretation:The mechanism for formation of product in indicated reaction should be written.

Concept introduction: The carbonyl bond is polar with partial postive charge on carbon and partial negative charge on oxygen as illustrated below.
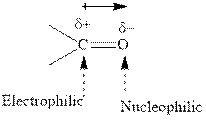
Thus it can undergo hydride addition at carbon and proton addition at oxygen. Certain reagents that are useful for such hydride addition at carbonyl carbon include
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry: Structure and Function
- 2. Provide a mechanism and the favored product(s) with the following reaction. Remember MeOH всион MeOHarrow_forwardCan someone show a detailed reaction mechanism for this problem?arrow_forwardWhat major product (from Figure #1) results from the following dehydration reaction (from Reaction #1)? Reaction #1 HO, H2SO4 heat Figure #1 compound A compound B compound C compound D Please click here if image does not display. In predicting the major product of boxed reaction #1, it is strongly recommended that you work out a complete mechanism on a piece of scratch paper. compound C compound A compound D compound Barrow_forward
- Show a complete arrow pushing reaction mechanism for the reaction below.arrow_forwardPlease answer the subparts 4, 5, 6 and 7. Please mention all the mechanism and needed reagents. Thanksarrow_forwardWrite the correct mechanism and major product for a), and indicate the reagent needed for b) to happen.arrow_forward
- 4) Provide the major product for each of the reactions shown below. Watch out for carbocation stabilities. NaOEt EtOH BrYY EtOH heatarrow_forwardis this an E1 or E2 mechanism for this reaction? What is the major product and step by step mechanism?arrow_forwardPlease provide a complete arrowing-pushing mechanism for the following reaction with the product and reagents shown! Please show each step clearly and separately.arrow_forward
- 7. Complete the following reaction and propose a mechanism.arrow_forwardFill in the missing reagents, conditions, intermediates or products in each box below.arrow_forwardThe transformation below takes place by two distinct reactions. Intermediate A is formed in the first reaction and then this goes on to the product in the second reaction. Provide a complete curved-arrow mechanism for all steps of both reactions.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

