
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
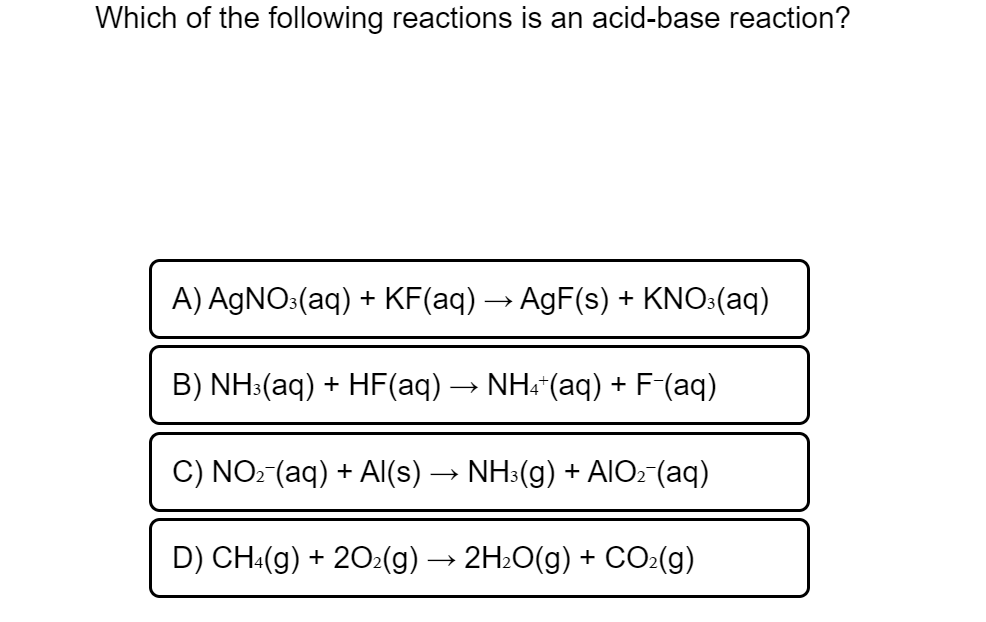
Transcribed Image Text:Which of the following reactions is an acid-base reaction?
A) AGNO:(aq) + KF(aq) → AgF(s) + KNO:(aq)
B) NH:(aq) + HF(aq) → NH:"(aq) +
-F(aq)
C) NO: (aq) + AI(s) → NH3(g) + AIO: (aq)
D) CH:(g) + 202(g) -
2H:O(g) + CO:(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Complete the balanced chemical reaction for the following weak base with a strong acid. In this case, write the resulting acid and base as its own species in the reaction. NH3(aq) + HCl(aq) – ->arrow_forwardWhich one of the reactions represents an ionization equation for a binary acid? O A) HC2H3O2(aq) --> H1+(aq) + C2H3O2 1 (aq) B) H2CO3(5) --- H+1, (aq) + CO3 (aq) C) NaOH (s) Na +1 (aq) + OH 1(aq) --> O D) HF(aq) --> H*(aq) + F¯ (aq)arrow_forward83. Determine the pH of each solution and classify it as acidic, basic, or neutral. (a) pOH = 8.5 = 4.2 (b) pOH (c) POH = 1.7 (d) pOH = 7.0arrow_forward
- Complete the following neutralization reaction between an acid and a base. Do not incluc not write coefficients of "1." x x, He Н, СО, + 2 КОН > 2H,0+K,CO3arrow_forwardThe pOH of an aqueous solution of 0.591 M phenol (a weak acid), C,H5OH, isarrow_forwardWhich of the following unbalanced reactions are acid-base reactions?Select all that are True. HCl(aq) + Al(s) → H2(g) + AlCl3(aq) H2O2(aq) → H2O(l) + O2(g) KCl(aq) + AgNO3(aq) → KNO3(aq) + AgCl(s) NaH2PO4(aq) + NaOH(aq) → Na2HPO4(aq) + H2O(l) Br2(l) + Cl2(g) → BrCl(g) Ba(OH)2(aq) + H2SO4(aq) → BaSO4(s) + H2O(l)arrow_forward
- (a) A 50.0 mL solution is prepared to be 1.29 M acetylsalicylic acid. In the first step, 8.55 mL of NaOH is titrated into the solution until the pH is exactly 5.0. What is the concentration of the titrant (NaOH)? (b) In the second step, enough 5.85 M nitric acid is added to the solution after the titration in part (a) is complete until the pH is one unit lower than the pKa of acetylsalicylic acid. What volume (mL) of nitric acid was added?arrow_forwardpOH of 10.81 What is the Molarity of hydroxide ions in the solution?arrow_forwardIdentify the acid, base, conjugate acid and conjugate base in the following reactions: HF (aq) + H,O (1) H;O* (aq) + F ¯(aq) CH;CH,NH, (aq) + H,O (1) CH;CH,NH;" (aq)+OH (aq)arrow_forward
- Write the formula for the conjugate base of each of the following acids: (a) CH,CICOOH, (b) HIO4, (c) H,PO4, (d) H,PO, (e) HPO, (f) H,SO,, (g) HSO,, (h) HIO3, (i) HSO,, (j) NH, (k) H,S, (1) HS, (m) HCIO. A58arrow_forwardThe pH of an aqueous solution of 0.506 M benzoic acid , C6H5COOH isarrow_forwardIf the pOH of a solution is 4.21, what is its pH?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY