
Concept explainers
(a)
Interpretation: Out of the two indicated brominated system the one molecule that would undergo
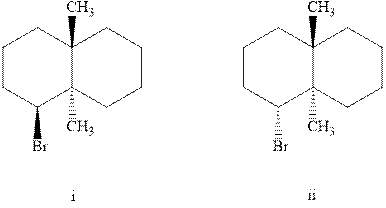
Concept introduction: Experimental evidence for
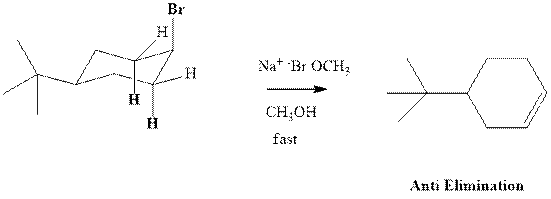
(b)
Interpretation:Whether anti or syn elimination occurs in the indicated reactions should be written.
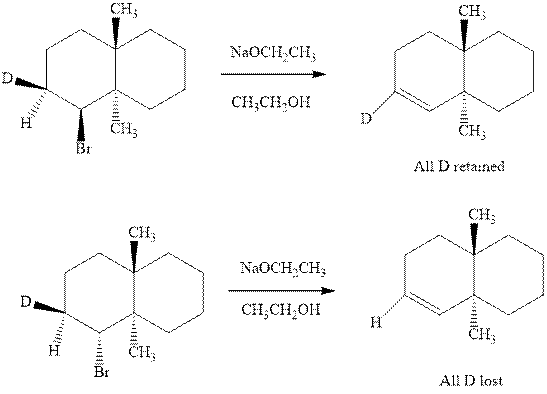
Concept introduction: Experimental evidence for
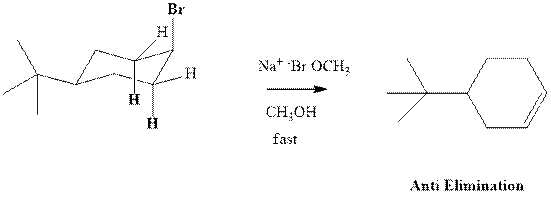
Trending nowThis is a popular solution!

Chapter 7 Solutions
Organic Chemistry: Structure and Function
- QUESTION structure of the final products A and B. Provide a complete mechanism with arrow pushing The two reactions below give slightly different products. Show the showing the intermediates and all resonance forms for one transformation. Explain the differences between the two reactions. HCI (cat) CH2CH3 CH3OH (excess) Зда HCI (cat) B CH2CH3 CH CH2OH (excess)arrow_forwardis a valuable synthetic intermediate because each of its Epichlorohydrin three carbons contains a reactive group. Following is the first step in its synthesis from propene. Propose a mechanism for this step. + Cl, 500°C Cl. + HCI 3-Chloropropene (Allyl chloride) Propenearrow_forwardDescribe the mechanism by which benzene is converted to bromobenzene (C6H5Br) on treatment with a mixture of bromine and iron (III) bromide (Br2/FeBr3). Your answer should illustrate how the electrophile (Br*) is formed from the Br₂/FeBr3 and how it is involved in the electrophilic substitution of the aromatic ring.arrow_forward
- Wittig reactions with the following -chloroethers can be used for the synthesis of aldehydes and ketones. (a) Draw the structure of the triphenylphosphonium salt and Wittig reagent formed from each chloroether. (b) Draw the structural formula of the product formed by treating each Wittig reagent with cyclopentanone. Note that the functional group is an enol ether or, alternatively, a vinyl ether. (c) Draw the structural formula of the product formed on acid-catalyzed hydrolysis of each enol ether from part (b).arrow_forwardPropose a mechanism to account for the formation of 3, 5-dimethyl- pyrazole from hydrazine and 2, 4-pentanedione. Look carefully to see what has happened to each carbonyl carbon in going from starting material to product.arrow_forwardChoose the best reagents from the list provided below for carrying out the following conversion. Match the reagent with the step number. HCl (aq), Zn(Hg) Br2, FeBr3 Na/NH3, -33 degrees C NBS, light KMnO4, H3O+ Mg metal, ether KOH, EtOH, heatarrow_forward
- A student wanted to try to make phenoxyacetic acid using hydroxyacetic acid (HOCH2COOH) and chlorobenzene. His plan was to use NaH to deprotonate both OH groups, and then the more basic alkoxy group would react with the chlorobenzene. Would this procedure work? Explain.arrow_forwardWhich of the following is an incorrect statement about the bromination of benzene by Br2 and FeBr3? O FeBr3 functions to increase the electrophilicity of Br2. O The carbanioni intermediate is resonance stabilized. O Formation of the sigma complex is the rate-determining step of the mechanism. O There are two carbon-containing intermediates in the mechanism. O The final step of the mechanism is loss of H+. adlanation pollo 8888850 Ber li S SAARISMA Waamarrow_forwardDraw the product of the reaction shown below. Ignore inorganic byproducts. Na2Cr2O7 H2O, CH3CO2H aarrow_forward
- The following reaction involves two sequential Heck reactions. Draw structural formu- las for each organopalladium intermediate formed in the sequence and show how the final product is formed. Note from the molecular formula given under each structural formula that this conversion corresponds to a loss of H and I from the starting material. Acetonitrile, CH,CN, is the solvent. 1% mol Pd(OAc), 4% mol Ph,P CH,CN C4H171 C4H16arrow_forwardb. Design the syntheses of the following three compounds, i, ii, and iii using the starting materials provided along with any reagents discussed in CHEM2401 and CHEM2402. The starting materials need to be transformed for the development of the reaction pathways and cannot be used directly to form the products. These are multiple step syntheses. Starting materials: ΌΗ مجھ ہم مجھ لةarrow_forwardDraw the product of the reaction shown below. Ignore inorganic byproducts. Na2Cr207 19 Acetone, H2SO4 Atoms, Bonds and Rings Draw or tap a nevarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

