
Concept explainers
(a)
Interpretation:
The two major substitution products of the following reaction should be drawn along with the mechanism for the formation.
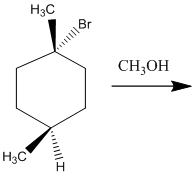
Concept Introduction:
The
The chemical reaction in which displacement of leaving group occurs by a nucleophile is known as nucleophilic substitution reaction.
The reaction between nucleophile (electron pair donor) and electrophile (electron pair acceptor) is known as nucleophilic substitution reaction. It is classified as SN1 and SN2 reaction.
Cis-Trans isomers are known as geometric isomers. In Cis isomers,
(b)
Interpretation:
The reaction mixture should be monitored which reveals an isomer of the starting material is generated as an intermediate along with its structure and its preparation.
Concept Introduction:
The chemical reaction in which one functional group is substituted by another functional group is known as substitution reaction.
The chemical reaction in which displacement of leaving group occurs by a nucleophile is known as nucleophilic substitution reaction.
The reaction between nucleophile (electron pair donor) and electrophile (electron pair acceptor) is known as nucleophilic substitution reaction. It is classified as SN1 and SN2 reaction.
Cis-Trans isomers are known as geometric isomers. In Cis isomers, functional groups are present on same side of the carbon chain whereas in Tans isomers, functional groups are present on different or opposite side of the chain.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: Structure and Function
- Draw out the mechanism to explain this reaction and predict the products that will resultarrow_forwardPlease provide the mechanism and reagents for this reactionarrow_forwardRefer to the reaction energy diagram to answer parts (a) through (e). (a) What is the rate-limiting STEP? (Select from 1, 2, 3, or 4) (b) The TRANSITION STATE for the fastest step is: (c) In the 3rd step which INTERMEDIATE structurally resembles the transition state according to the Hammon postulate? (d) Which STEP NUMBER is endergonic? (e) The number of intermediates in the overall reaction is:arrow_forward
- Complete the following reaction diagram by giving the reaction condition or the missing product. The reaction mechanism is not required.arrow_forwardProvide mechanisms for the two reactions.arrow_forward(a) Propose a mechanism for reaction A, which is a substitution reaction. (b) Explain why reaction B does not lead to a similar substitution.arrow_forward
- Many primary amines, RNH2, where R is a carboncontainingfragment such as CH3, CH3CH2, and so on,undergo reactions where the transition state is tetrahedral.(a) Draw a hybrid orbital picture to visualize thebonding at the nitrogen in a primary amine (just use a Catom for “R”). (b) What kind of reactant with a primaryamine can produce a tetrahedral intermediate?arrow_forwardModify the structure to represent the organic product, if any, expected from each fo the oxidation reactions. Note that (O) represens an oxidizing agent, such as potassium dichromate, and that (O) is present in excess.arrow_forwardWrite the mechanism for these two reactions. include resonance structures in the mechanism.arrow_forward
- The following reaction, which is discussed in Chapter 8, is an example of a unimolecular nucleophilic substitution (Sn1) reaction. It consists of the four elementary steps shown here. For each step (i-iv), (a) identify all electron-rich sites and all electron-poor sites, (b) draw in the appropriate curved arrows to show the bond formation and bond breaking that occur, and (c) name the elementary step. (i) H-OCH3 OH + H-OCH3 H. H (ii) ©CH2 + H,O (iii) ©CH2 CH3 + HO-CH3 (iv) CH3 CH3 + H2O-CH, + НО—СНЗ foarrow_forwardWrite the reaction mechanism for the reaction on the image and show the main organic product. Please include all of the steps in the explanation.arrow_forwardThe reaction shown here yields three different nucleophilic substitution products that are constitutional isomers of one another. (a) Does this suggest an SN1 or Sn2 mechanism? (b) Draw the mechanism for the formation of each of these products. CH3CH2OH Brarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





