
(a)
Interpretation: The mistake in the mechanism of indicated reaction should be pointed out and correction should be proposed.

Concept introduction:Carbocation formation is relatively slower than acid-base reactions. Carbocations generated from
Unimolecular substitution or
A general

(b)
Interpretation: The mistake in the mechanism of indicated reaction should be pointed out and correction should be proposed.
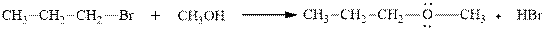
Concept introduction:Carbocation formation is relatively slower than acid-base reactions. Carbocations generated from alkyl halides have two fates; they can be either trapped by nucleophiles to give substitution product or may deprotonate to yield a small amount of alkene.
Unimolecular substitution or
A general

(c)
Interpretation: The mistake in the mechanism of indicated reaction should be pointed out and correction should be proposed.
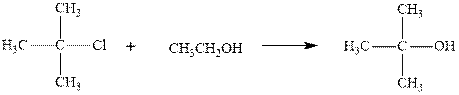
Concept introduction: Carbocation formation is relatively slower than acid-base reactions. Carbocations generated from alkyl halides have two fates; they can be either trapped by nucleophiles to give substitution product or may deprotonate to yield a small amount of alkene.
Unimolecular substitution or
A general

Trending nowThis is a popular solution!

Chapter 7 Solutions
Organic Chemistry: Structure and Function
- ns 1. Acid-catalized rearrangement reactions of simple alcohols are quite common. Based on the mechanism shown in the background, can you predict the product and mechanisms for the reation of a strong acid and isopropyl alcohol? Answer: OH OH2 H* H20 H+ CH3 CH CH CH. CH CH3 CH3 CH3 CH3 CH3 H2C=C H. 2. Why is acetic acid a better acid catalyst than hydrogen chloride for this carbocation rearrangement?arrow_forwardGiven the following substitution reaction, what would the effect be of changing the solvent from CH3OH to (CH3)2S=O? CH₂(CH₂)4 CH₂-Br Select one: + Na* OH CH3(CH₂)4 CH₂-OH + Na Br A. The rate of the reaction would decrease. O B. The rate of the reaction would increase. O C. The rate of the reaction would not change.arrow_forwardSelect the correct product of the following reaction series. 1. NaOEt, EtOH 2. Br EtO NHAC OEt 3. H3O+, 100°C ? H₂N. CO₂H HẠN_CO,H HẠN_CO,H Br I II IIIarrow_forward
- Which of the reactions shown below are correct? Y On H 1.) Na OEt O 2.) CH 3 1.) LDA OCH3 2.) CH3CH₂Br PH CHO 1.) LDA 2.) CH3CH₂Br 여 1.) NaOH, H₂O 2.) 12 Ph CH3 двосна OCH 3 CH₂arrow_forwardIdentify the product(s) in the reaction below. 2H20→ 2H2 + 02 O A. H20 and 02 B. H20 C. H20 and H2 D. H2 and 02arrow_forwardWhich reagent(s) will complete the following reaction? NH2 1. NaNO2, H2S04 2. D3PO2 1. HNO3 2. D20 1. NaNO2, D2SO4 2. H3PO2 1. NaNO2, H2SO4 2. LIAID4 D30* II II IV V O a. I O b. II О с. Ш O d. IV О е. Varrow_forward
- 1a. What is the mechanism for reaction 2? 1b. What is the mechanism for reaction 3? a Electrophilic addition b Electrophilic substitution c Radical substitution d Rearrangement 2. What reagents are needed in reaction 3? a 3H2, Rh (with high pressure) b H2, PtO2 c KMnO4, H+ d Br2, uvarrow_forward4. Write the product and the mechanism for reaction. Write the names of starting material and product according IUPAC or trivial nomenclature. H+ JOH CH₂ OH OHarrow_forwardIn each reaction box, place the best reagent and conditions from the list. 1) 2) Answer Bank H₂O₂ Br₂ HBr (CH3)3 CO CH3CH₂O BH3/THF 03 CH COO NaBH4arrow_forward
- II. Simple Synthesis Fill in the missing reagents in the reaction schemes shown. The transformations might require more than one step. obou 0-0 CI H H ļ Brarrow_forwardwrite the mechanism of each reaction R-X Base →A- HOAC 3 14. HOAC NaBHy R. Carrow_forward10. Write a detailed mechanism for the following reaction. Draw structures of the expected products. Label the MAJOR and MINOR products. Indicate the slow and fast steps. H-Br A + B + Carrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





