
Concept explainers
(a)
Interpretation:Whether
Concept introduction: Carbocations generated from alkyl halides have two fates; they can be either trapped by nucleophiles to give substitution product or may deprotonate to yield a small amount of alkene.
If the leaving group present eliminates along with a proton in absence of any strong nucleophile the double bond formation might occur. Such reactions are known as two step unimolecuar elimination, abbreviated as
(b)
Interpretation:Whether
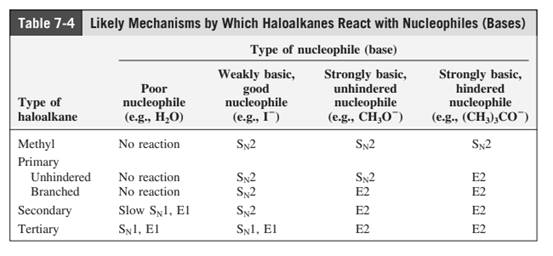
Concept introduction: The most likely mechanisms for different kinds of alkyl halides with various nucleophiles is given as follows:
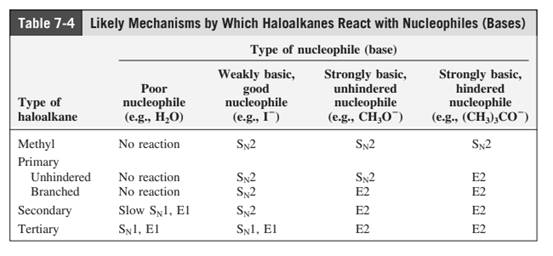
(c)
Interpretation:Whether
Concept introduction: The most likely mechanisms for different kinds of alkyl halides with various nucleophiles is given as follows:
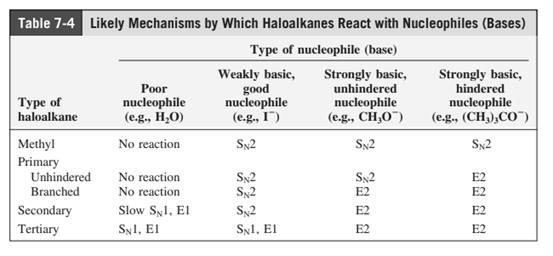
(d)
Interpretation:Whether
Concept introduction: The most likely mechanisms for different kinds of alkyl halides with various nucleophiles is given as follows:
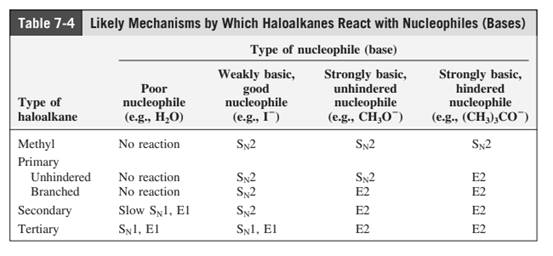
(e)
Interpretation:Whetherbelow reagent will give
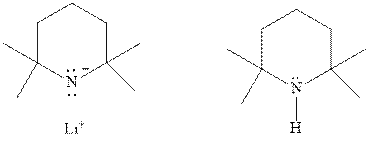
Concept introduction: The most likely mechanisms for different kinds of alkyl halides with various nucleophiles is given as follows:
(f)
Interpretation:Whether
Concept introduction: The most likely mechanisms for different kinds of alkyl halides with various nucleophiles is given as follows:
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: Structure and Function
- 4A2 Give the major products of the following reactions and explain COOCH3 + H H3COOC Harrow_forward4A1 Give the major products of the following reactions and explain +arrow_forward3. For each of the following reactions, fill in the missing products, reactants, or reagents as appropriate. Account for stereochemistry when appropriate. CI Br KCN CH3OH Ph CH3OH (а) (b) CH3 CH3 KO'Bu 'BUOH Brarrow_forward
- 22. Explain why the following compound cannot be converted to a Grignard reagent. How would you resolve this problem? O & Br O A dimer would form; treat C=0 with diol in acid Mg would react with C=0; treat C=0 with diol in acid A dimer would form; treat Br with diol in acid O Mg would react with C=0; treat Br with diol in acid MgBrarrow_forward18. Identify the products from each step in the following synthesis sequence of reactions. Show the structure of all reactants and products from each step. (a) 3-methyl-1-pentanol + PBr3 --------> A (b) A + Mg/ether-------->B (c) B + 1.CH3CH2C(O)H/2.H3O+ -------> C (d) C + Na2Cr2O7/H2SO4, H2O -----> Darrow_forward(b) Predict the suitable solvent (H2O or CH3COCH3) to increase the reaction of bromopropane (CH3CH2CH2B1) with sodium hydroxide (NaOH). Two reactions are shown below: NaOH, 55 °C CH;CH,CH,Br CH;CH,CH,OH + NaBr H,O (i) NaOH, 55 °C CH;CH,CH,Br CH;CH,CH,OH NaBr H,C CH (ii)arrow_forward
- n-Pentanol (CH3CH2CH2CH2CH2OH) and 2-methylbutan-2-ol (CH3CH2C(CH3)2OH) are converted to their corresponding alkyl chorides on being reacted with hydrogen chloride. (a) Write out an equation for each reaction (b) Assign each the appropriate symbol (SN1 or SN2) (c) Write a suitable mechanism for each reactionarrow_forwardPredict the major product obtained from each of the following reactions. Show the stereochemistry where pertinent. The starting material for the second step reaction will be the product obtained from first step reaction. Use only major product obtained from the first step to proceed into the second step. (4a) OH (4b) Br Br 1. TSCI/Pyridine 2. NaOEt t-BuOK HBr (1 equiv.) 40 °C HCl (1 equiv.) 0 °Carrow_forwardIdentify the best reagent(s) for this reaction. (CH3)2CHCH2C=CH (CH,)2CHCH,CH2CHarrow_forward
- 10). Give the major product of the following reaction. (A) (D) OCH 3 1). CH3NH₂ OH (B) CH3 (E) 2). LiAlH4, then H₂O NH–CH3 HN CH₂ H ? -CH3 N-CH3 CH3arrow_forward3 a) State Zaitsev's rule and show the structures elimination product(s) of the following compounds including conditions: [2+8] Br Brarrow_forwardWhich set of reagents would be appropriate to synthesize bromobenzene from benzene? 1. HNO3 in H2SO4; 2. H2Cr04 and heat 1. H2CrO4 and heat; 2. H2 Pd/C Br2 and FeBr3 1. CH3CH2CHCI and AICI3; 2. H204 and heat 4B12 and Fe Heat and Br2 Br2, HCIarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





