
Concept explainers
(a)
Interpretation:
The products of the following reaction should be drawn along with the mechanism for the formation. Whether the given transformation is faster in polar, aprotic solvent in comparison to polar, protic solvent should be determined.
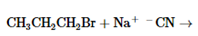
Concept Introduction:
The
The chemical reaction in which displacement of leaving group occurs by a nucleophile is known as nucleophilic substitution reaction.
The reaction between nucleophile (electron pair donor) and electrophile (electron pair acceptor) is known as nucleophilic substitution reaction. It is classified as SN1 and SN2 reaction.
The solvents which are capable of forming hydrogen bonds due to the presence of at least one hydrogen linked with electronegative atom is known as polar protic solvents whereas the solvents in which no hydrogen atoms are linked with electronegative atom and also incapable of hydrogen bonding is known as
(b)
Interpretation:
The products of the following reaction should be drawn along with the mechanism for the formation. Whether the given transformation is faster in polar, aprotic solvent in comparison to polar, protic solvent should be determined.
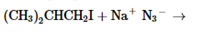
Concept Introduction:
The chemical reaction in which one functional group is substituted by another functional group is known as substitution reaction.
The chemical reaction in which displacement of leaving group occurs by a nucleophile is known as nucleophilic substitution reaction.
The reaction between nucleophile (electron pair donor) and electrophile (electron pair acceptor) is known as nucleophilic substitution reaction. It is classified as SN1 and SN2 reaction.
The solvents which are capable of forming hydrogen bonds due to the presence of at least one hydrogen linked with electronegative atom is known as polar protic solvents whereas the solvents in which no hydrogen atoms are linked with electronegative atom and also incapable of hydrogen bonding is known as polar aprotic solvents
(c)
Interpretation:
The products of the following reaction should be drawn along with the mechanism for the formation. Whether the given transformation is faster in polar, aprotic solvent in comparison to polar, protic solvent should be determined.
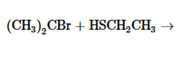
Concept Introduction:
The chemical reaction in which one functional group is substituted by another functional group is known as substitution reaction.
The chemical reaction in which displacement of leaving group occurs by a nucleophile is known as nucleophilic substitution reaction.
The reaction between nucleophile (electron pair donor) and electrophile (electron pair acceptor) is known as nucleophilic substitution reaction. It is classified as SN1 and SN2 reaction.
The solvents which are capable of forming hydrogen bonds due to the presence of at least one hydrogen linked with electronegative atom is known as polar protic solvents whereas the solvents in which no hydrogen atoms are linked with electronegative atom and also incapable of hydrogen bonding is known as polar aprotic solvents
(d)
Interpretation:
The products of the following reaction should be drawn along with the mechanism for the formation. Whether the given transformation is faster in polar, aprotic solvent in comparison to polar, protic solvent should be determined.

Concept Introduction:
The chemical reaction in which one functional group is substituted by another functional group is known as substitution reaction.
The chemical reaction in which displacement of leaving group occurs by a nucleophile is known as nucleophilic substitution reaction.
The reaction between nucleophile (electron pair donor) and electrophile (electron pair acceptor) is known as nucleophilic substitution reaction. It is classified as SN1 and SN2 reaction.
The solvents which are capable of forming hydrogen bonds due to the presence of at least one hydrogen linked with electronegative atom is known as polar protic solvents whereas the solvents in which no hydrogen atoms are linked with electronegative atom and also incapable of hydrogen bonding is known as polar aprotic solvents
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: Structure and Function
- The reaction shown below is carried out in two stages. In the first, the two reactants are combined in tetrahydrofuran (THF) solvent to form a neutral adduct. Aqueous acid is then added to hydrolyze this initial adduct, giving the final product. N(CH3)2 I. H a For the reaction shown above, draw the major organic product having at least one carbonyl group. • You do not have to consider stereochemistry. Pis 85 1. THF 2. H3O+ n [ ]#arrow_forwardWhat explains why many aldehydes and ketones can undergo self-condensation reactions in basic conditions? The alpha carbon can lose a proton and act like a nucleophile and the carbonyl carbon is an electrophile. The alpha carbon can gain a proton and act like an electrophile and the carbonyl carbon is a nucleophile. The oxygen of the carbonyl group can attack the carbon of the carbonyl group. Only esters can undergo self-condensation reactions.arrow_forwardWhy must dehydration reactions of alcohols be carried out under acid catalysis? O 1) the hydroxide group is a poor leaving group and must be protonated to 1) generate a better leaving group. 2) the hydroxide group is a great leaving group and does not need to be protonated to generate a better leaving group. O 3) the acid isn't necessary for the reaction. O4) None of these are correct answers.arrow_forward
- Predict the b-elimination product(s) formed when each bromoalkane is treated with sodium ethoxide in ethanol. If two or more products might be formed, predict which is the major productarrow_forwardWhen propene reacts with gaseous hydrogen bromide, HBr, two products, 1-bromopropane and 2-bromopropane are formed. The reaction is a two-step process in which the electrophilic attack occurs in the first step. Identify the electrophile in this reaction Draw a diagram showing the first step of the reaction that leads to the production of 2-bromopropane.arrow_forwardProvide a detailed step-by-step mechanisms for the reaction shown. CH3CH,SH + Br, + NaOHarrow_forward
- Peroxides are often added to free-radical reactions as initiators because the oxygen–oxygen bond cleaves homolytically rather easily. For example, the bond-dissociation enthalpy of the O¬O bond in hydrogen peroxide (H¬O¬O¬H) is only 213 kJ>mol (51 kcal>mol). Give a mechanism for the hydrogen peroxide-initiated reaction of cyclopentane with chlorine. The BDE for HO¬Cl is 210 kJ>mol (50 kcal>mol).arrow_forwardWrite a mechanism that accounts for the formation of ethyl isopropyl ether as one of the products in the following reaction. CI OEt HCI EtOH Write the mechanism for step one of this reaction. Show lone pairs and formal charges. Only the acidic hydrogen should be drawn out with a covalent bond. Write the mechanism for step two of this reaction (where the product of step one reacts with the solvent, ethanol). Show lone pairs and formal charges. Only the acidic hydrogen should be drawn out with a covalent bond. Write the mechanism for the last step of this reaction (formation of ethyl isopropyl ether). Show lone pairs and formal charges. Only the acidic hydrogen should be drawn out with a covalent bond. CI will act as the base in this reaction.arrow_forwardWhen ethoxybenzene is treated with a mixture of nitric acid and sulfuric acid, two products are obtained, each of which has the molecular formula C8H9NO3. For the mechanism, draw the curved arrows as needed. Include lone pairs and charges in your answer. Do not draw out any hydrogen explicitly in your products. Do not use abbreviations such as Me or Ph.arrow_forward
- Choose all of the following reaction mechanisms that are possible from the reaction between ethyl bromine and the cyanide ion. O The lone pair on the carbon atom in the cyanide ion attacks the carbon adjacent to bromine and the bromine acts as the leaving group. The electrons of the triple bond in the cyanide ion attack the carbon adjacent to bromine and a methyl group acts as the leaving group. V The lone pair on the nitrogen atom in the cyanide ion attacks the carbon adjacent to bromine and the bromine acts as the leaving group. The electrons of the double bond in the cyanide ion attack the carbon adjacent to bromine and a methyl group acts as the leaving group. The electrons of the double bond in the cyanide ion attack the carbon adjacent to bromine and the bromine acts as the leaving group. The lone pair on the nitrogen atom in the cyanide ion attacks the carbon not adjacent to bromine and the bromine acts as the leaving group. The lone pair on the carbon atom in the cyanide ion…arrow_forwardAn electron-deficient carbon atom reacts with a nucleophile, symbolized as: Nu−. Define this ?arrow_forwardA graduate student was studying enzymatic reductions of cyclohexanones when she encountered some interesting chemistry. When she used an enzyme and NADPH to reduce the following ketone, she was surprised to find that the product was optically active. She carefully repurified the product so that no enzyme, NADPH, or other contaminants were present. Still, the product was optically active. If this reaction could be accomplished using H2 and a nickel catalyst, would the product be optically active? Explain.arrow_forward
