
Concept explainers
Interpretation:
The following carbocation should be ranked in decreasing order of stability.
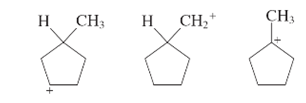
Concept Introduction:
The molecule in which carbon atom is linked with three bonds and carries one formal positive charge is known as carbocation’s. In these molecules, six electrons are present in their valence shell which makes them electron deficient. This makes them unstable electrophiles which bind with nucleophile (electron rich) to form new bond.
The stability ranking of carbocation can be determined on the basis of number of carbon atoms linked with carbocation.
Tertiary carbocation: Carbocation is linked with three carbon atoms.
Secondary carbocation: Carbocation is linked with two carbon atoms.
Primary carbocation: Carbocation is linked one carbon atom.
The order is given as:
Tertiary
(More carbon atoms)
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: Structure and Function
- Rank the alkenes shown in the ball-and-stick models (A–C) in order of increasing stability.arrow_forwardRank the following carbocations in order of decreasing stability, putting the most stable first. II Multiple Choice |> || > II Il >l> II III > T> || III > || > Tarrow_forwardRank the following alkenes in order of increasing stability (least to most stable) I II III IVarrow_forward
- Rank the following alkenes from the most to the least stable.arrow_forwardDraw the alkene that would react with the reagent given to account for the product formed. ? + H₂O H₂SO4 CH3 CH3 CHCCH3 OH CH3 • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. Sn [F ChemDoodlearrow_forwardWhich carbocation would be least stable? II + + III IV Varrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





