
(a)
Interpretation:
The products of the following reaction should be drawn along with the mechanism for the formation. Whether the product arises through SN1 or SN2 process should be indicated.
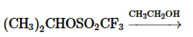
Concept Introduction:
The
The chemical reaction in which displacement of leaving group occurs by a nucleophile is known as nucleophilic substitution reaction.
The reaction between nucleophile (electron pair donor) and electrophile (electron pair acceptor) is known as nucleophilic substitution reaction. It is classified as SN1 and SN2 reaction.
The reaction which takes place in one step is known as SN2 reaction whereas reaction which takes place in two steps is known as SN1 reaction.
(b)
Interpretation:
The products of the following reaction should be drawn along with the mechanism for the formation. Whether the product arises through SN1 or SN2 process should be indicated.
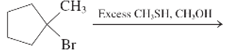
Concept Introduction:
The chemical reaction in which one functional group is substituted by another functional group is known as substitution reaction.
The chemical reaction in which displacement of leaving group occurs by a nucleophile is known as nucleophilic substitution reaction.
The reaction between nucleophile (electron pair donor) and electrophile (electron pair acceptor) is known as nucleophilic substitution reaction. It is classified as SN1 and SN2 reaction.
The reaction which takes place in one step is known as SN2 reaction whereas reaction which takes place in two steps is known as SN1 reaction.
(c)
Interpretation:
The products of the following reaction should be drawn along with the mechanism for the formation. Whether the product arises through SN1 or SN2 process should be indicated.
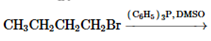
Concept Introduction:
The chemical reaction in which one functional group is substituted by another functional group is known as substitution reaction.
The chemical reaction in which displacement of leaving group occurs by a nucleophile is known as nucleophilic substitution reaction.
The reaction between nucleophile (electron pair donor) and electrophile (electron pair acceptor) is known as nucleophilic substitution reaction. It is classified as SN1 and SN2 reaction.
The reaction which takes place in one step is known as SN2 reaction whereas reaction which takes place in two steps is known as SN1 reaction.
(d)
Interpretation:
The products of the following reaction should be drawn along with the mechanism for the formation. Whether the product arises through SN1 or SN2 process should be indicated.
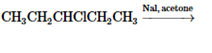
Concept Introduction:
The chemical reaction in which one functional group is substituted by another functional group is known as substitution reaction.
The chemical reaction in which displacement of leaving group occurs by a nucleophile is known as nucleophilic substitution reaction.
The reaction between nucleophile (electron pair donor) and electrophile (electron pair acceptor) is known as nucleophilic substitution reaction. It is classified as SN1 and SN2 reaction.
The reaction which takes place in one step is known as SN2 reaction whereas reaction which takes place in two steps is known as SN1 reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: Structure and Function
- Which alcohols can be prepared as a single product by hydroboration– oxidation of an alkene? Which alcohols can be prepared as a single product by the acid-catalyzed addition of H2O to an alkene?arrow_forwardWrite down the common (not IUPAC) names of the organic molecules that would be released if this molecule were hydrolyzed: CH,—O—C—(CH2)—CH=CH-CH,—CH=CH—(CH2) — CH3 CH-0- -(CH2)–CH=CH(CH2)CH3 O CH,—O-C=(CH2)=CH=CH(CH2)—CH3 Separate each name with a comma and a space. You will find useful information in the ALEKS Data resource. type your answer...arrow_forwardDraw structures of compounds that fit the following descriptions: a) An , unsaturated ketone, C9H8O b) An diketone c) An aromatic ketone, C9H10O d) A diene aldehyde, C7H8Oarrow_forward
- 5A In the following reactions, mixtures of alkenes and ethyl ethers are formed. Draw their structures. Explain which is or are likely to be the main product(s) in each reaction. In case of formation of two isomers of alkenes, explain which is formed in greater proportion CH3 ofi H3C- -Br CH3 EtOHarrow_forward0 CH₂ → ?? If the above compound is subjected to combustion, what are the products? ethanoic acid +2-phenyl-ethene-1-ol 15CO₂ (g) + 5H₂0 (g) O 2-phenyl-ethyl-ethanoate + H₂O 20CO₂ (g) + 10H₂O(g) →?? CH, if the above compound was treated with H and H₂O what product will form?arrow_forward问题5 5分 Which of the following describe the major product(s) of this reduction reaction (you may assume excess LIAIH4 is used)? 1. LİAIH4 2. H20 rning primary alcohol secondary alcohol tertiary alcohol aldehyde ketone esterarrow_forward
- describe how the following conversions could be carried out. In each case give reagents and conditions of the reactions and the structures of the products. a) CH2=CH2 -> CH2 COOH b) ethanol -> 2hydroxypropanoic acidarrow_forward5B In the following reactions, mixtures of alkenes and ethyl ethers are formed. Draw their structures. Explain which is or are likely to be the main product(s) in each reaction. In case of formation of two isomers of alkenes, explain which is formed in greater proportion CH3 CH3 H3C-C H -Br CH3 EtOHarrow_forwardDraw the organic product you would expect to isolate from the nucleophilic substitution reaction between the molecules shown. Note: You do not need to draw any of the side products of the reaction, only the substitution product. + H₂O Cl + ☑ 5 C Click and drag to start drawing a structure.arrow_forward
- SN2 reaction is an example of a nucleophilic substitution reaction. Imagine that 1-iodobutane reacts with 4-methylheptan-1-ol under basic conditions. Draw the structure of the two reactants.arrow_forwardIn an advanced synthetic chemistry experiment, a researcher prepares a compound, ZY-7, by reacting a ketone (C5H100) with hydroxylamine (NH2OH), followed by heating in the presence of an acid catalyst. The resulting compound, ZY-7, is then treated with a solution of sodium nitrite (NaNO2) and hydrochloric acid (HCI) at low temperature. Identify the class of compound that ZY-7 most likely belongs to after this series of reactions." A) Amide B) Oxime C) Nitro compound D) Diazonium salt E) Ester Don't use chatgpt please provide valuable answerarrow_forwardA compound with formula C7H12O is treated with sodium borohydride in methanol to yield 2,2-dimethylcylopentanol. Write a reaction scheme showing the structures of the reactant, the reagents, and the product. Will the product be optically active? Explain.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole



