
(a)
Interpretation: The explanation for poor nucleophilicity of the solvent should be suggested.
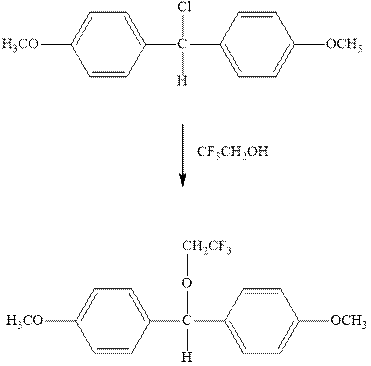
Concept introduction:Carbocation formation is relatively slower than acid-base reactions. Carbocations generated from
Bimolecular substitution or
A general
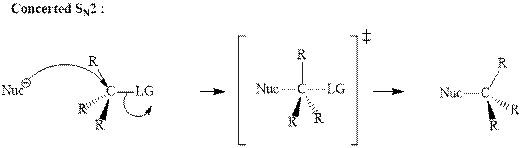
Unimolecular substitution or
A general

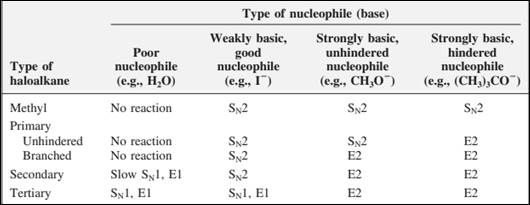
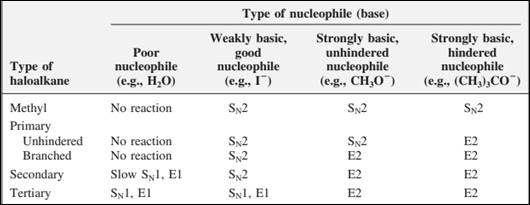
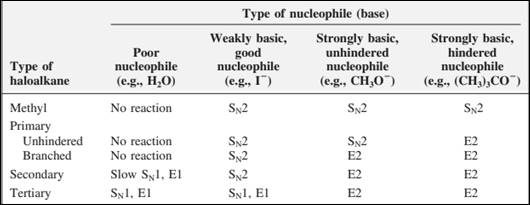
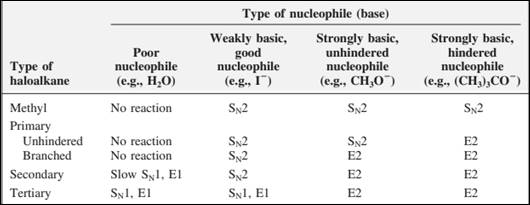
The most likely mechanisms for different kinds of alkyl halides reactions with various nucleophiles is given as follows:
(b)
Interpretation: The relative rates of the two steps should be found and compared to usual
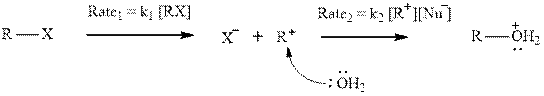
Concept introduction: Carbocation formation is relatively slower than acid-base reactions. Carbocations generated from alkyl halides have two fates; they can be either trapped by nucleophiles to give substitution product or may deprotonate to yield a small amount of alkene.
Bimolecular substitution or
A general
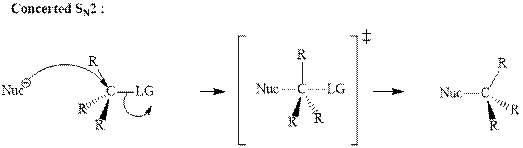
Unimolecular substitution or
A general

The most likely mechanisms for different kinds of alkyl halides reactions with various nucleophiles is given as follows:
(c)
Interpretation: The manner carbocation stability and decreasing solvent nucleophilicity might affect the relative magnitudes of
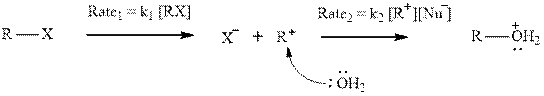
Concept introduction: Carbocation formation is relatively slower than acid-base reactions. Carbocations generated from alkyl halides have two fates; they can be either trapped by nucleophiles to give substitution product or may deprotonate to yield a small amount of alkene.
Bimolecular substitution or
A general
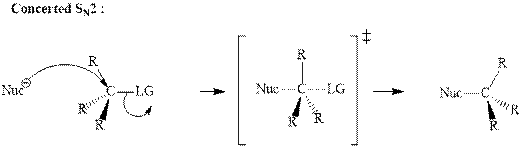
Unimolecular substitution or
A general

The most likely mechanisms for different kinds of alkyl halides reactions with various nucleophiles is given as follows:
(d)
Interpretation: The complete mechanism for the indicated reaction should be written.
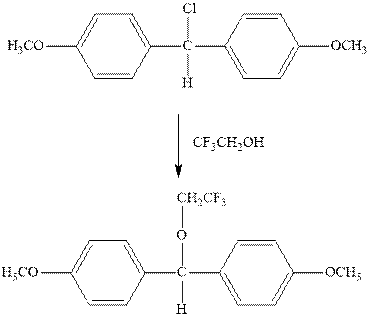
Concept introduction: Carbocation formation is relatively slower than acid-base reactions. Carbocations generated from alkyl halides have two fates; they can be either trapped by nucleophiles to give substitution product or may deprotonate to yield a small amount of alkene.
Unimolecular substitution or
A general

The most likely mechanisms for different kinds of alkyl halides reactions with various nucleophiles is given as follows:
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: Structure and Function
- Solvolysis of the following bicyclic compound in acetic acid gives a mixture of products, two of which are shown. The leaving group is the anion of a sulfonic acid, ArSO3H. A sulfonic acid is a strong acid, and its anion, ArSO3, is a weak base and a good leaving group. Propose a mechanism for this reaction.arrow_forwardWrite a mechanism that accounts for the formation of ethyl isopropyl ether as one of the products in the following reaction. CI OEt HCI EtOH Write the mechanism for step one of this reaction. Show lone pairs and formal charges. Only the acidic hydrogen should be drawn out with a covalent bond. Write the mechanism for step two of this reaction (where the product of step one reacts with the solvent, ethanol). Show lone pairs and formal charges. Only the acidic hydrogen should be drawn out with a covalent bond. Write the mechanism for the last step of this reaction (formation of ethyl isopropyl ether). Show lone pairs and formal charges. Only the acidic hydrogen should be drawn out with a covalent bond. CI will act as the base in this reaction.arrow_forwardPredict the product of the following epoxide ring opening and provide a mechanism for its formation. Be sure to include stereochemistry.arrow_forward
- When the alkyl bromides (listed here) were subjected to hydrolysis in a mixture of ethanol and water (80% EtOH/20% H2O) at 55 °C, the rates of the reaction showed the following order: (CH3)3CBR > CH3Br > CH3CH2Br > (CH3)2CHBR Provide an explanation for this order of reactivity.arrow_forwardSketch the reaction mechanism (including the final product) corresponding to the following description. Be prepared to explain the mechanism to your partner. 2-bromo-4-methylpentane is being reacted with sodium cyanide in diethyl ether (set up the reaction – next you will get a description of the mechanism to draw). A lone pair of electrons from the cyanide attacks the carbon bonded to bromine, forming a new carbon-carbon bond. At the same time, the carbon-bromine bond breaks with the electron pair from the carbon-bromine bond moving toward the bromine to form a bromide ion. This will form the product 2,4-dimethylpentanenitrile and sodium bromide. is this reaction following an SN1 or SN2 mechanism? Write out the rate law for this reaction.Draw a reaction coordinate diagram for this reaction.arrow_forward2,3-Dimethylbutane reacts with bromine in the presence of light to give a mono brominated product. The further reaction gives a good yield of a dibrominated product. Predict the structures of these products, and propose a mechanism for the formation of the mono brominated product.arrow_forward
- Alkynes do not react directly with aqueous acid as do alkenes, but will do so in the presence of mercury(II) sulfate as a Lewis acid catalyst. The reaction occurs with Markovnikov regiochemistry, so the OH group adds to the more highly substituted carbon and the H adds to the less highly substituted carbon. The initial product of the reaction is a vinyl alcohol, also called an enol. The enol immediately rearranges to a more stable ketone via tautomerization. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions -X티 Hö: H-O -CH3 -CH3 H30*arrow_forwardCyclohexanone + (CH₃)₂NH and continue with catalyze by H+. Predict the product of this reactionarrow_forwardWrite the synthesis mechanism for the reaction given below and demonstrate all the steps required to obtain the product in the reaction given below, using the appropriate reagents.arrow_forward
- Propose a mechanism for the conjugate addition of a nucleophile (Nuc:-) to acrylonitrile(H2C“CHCN) and to nitroethylene. Use resonance forms to show how the cyano andnitro groups activate the double bond toward conjugate addition.arrow_forwardSuggest mechanisms for the following reactions, both of which involve radical intermediates: (b) (c) Br. MeO MeO CO₂Et N-NO Bu3SnH, AIBN heat hv HO `N MeO MeO NH CO₂Etarrow_forwardPredict the products and mechanisms of the following reactions. When more than oneproduct or mechanism is possible, explain which are most likely. isobutyl chloride + AgNO3 in ethanol>waterarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
