
Concept explainers
Interpretation: The most likely mechanism of below reaction should be written. Also, role of strong acid should be determined.
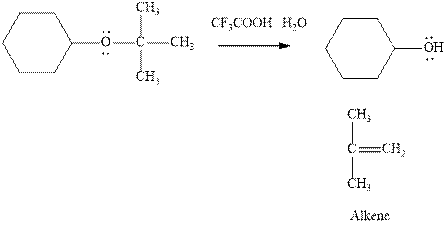
Concept introduction: Carbocations generated from
If the leaving group present eliminates along with a proton in absence of any strong nucleophile the double bond formation might occur. Such reactions are known as two step unimolecular elimination, abbreviated as
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: Structure and Function
- Following is the structure of miconazole, the active antifungal agent in a number of over-the-counter preparations, including Monistat, that are used to treat vaginal yeast infections. One of the compounds needed for the synthesis of miconazole is the trichloro derivative of toluene shown on its right. (a) Show how this derivative can be synthesized from toluene. (b) How many stereoisomers are possible for miconazole?arrow_forwardComplete the following reaction scheme. Give all product(s) and indicate major or minor and any relevant stereochemistry (d) dil HCl (e) ОН Conc H2SO4 heat (f) HBrarrow_forwardIII) Propose a reasonable, selective synthesis of the compound below from benzene and any other reagents with four or fewer carbon atoms. If a proposed EAS reaction gives multiple regioisomers, draw them all and indicate which will be used in subsequent reactions. ZONarrow_forward
- provide appropriate reagents or product for the following example if you can show work I'd appreciate it!!! OMe OMearrow_forwardPropose a reasonable mechanism for the following reaction.arrow_forwardProvide an arrow pushing mechanism for the following reaction. Include protons and each step clearly indicated. Predict the stereochemisty of the product.. Ph H + H3C CH3 + Ph2S Pharrow_forward
- Propose a reasonable mechanism to explain the following Reactions.arrow_forwardHow will you synthesize cyclohexanecarboxaldehyde (cyclohexylmethanal) from the following reagents? (There are no restrictions on the reagents or the number of steps). (a) Cyclohexanone (b) Ethynylcyclohexane (c) Methyl cyclohexylformate (Remember: Formic acid is the IUPAC recognized name for Methanoic acid) (d) Cyclohexanecarboxylic acid (Cyclohexylmethanoic acid) (e) Vinylcyclohexanearrow_forwardProvide the missing reagents to complete the reactions below, if there is more than one step required, be sure to indicate appropriately.arrow_forward
- Write a detailed mechanism for the following reaction. KCN HCNarrow_forwardComplete the Reaction below and provide the following (i) a detailed stepwise mechanism to account for the product(s), including all steps involved (ii) Clearly show all the resonance structures and intermediates formed leading to the product. OH acetic anhydridearrow_forwardThe compound to the right was treated under the following two conditions. Give the major product(s) of each reaction. If applicable, assume rearrangements will Br осcur. a) Left at 25°C overnight in ethanol b) Refluxed in boiling ethanol overnightarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
