
Concept explainers
(a)
Interpretation: The two main products of the indicated reaction should be drawn.
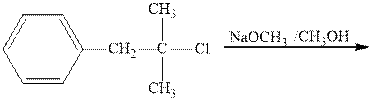
Concept introduction:Carbocations generated from
If the leaving group present eliminates along with a proton in absence of any strong nucleophile the double bond formation might occur. Such reactions are known as two-step unimolecuar elimination, abbreviated as
(b)
Interpretation: The mechanism I should be identified.
Concept introduction: Carbocations generated from alkyl halides have two fates; they can be either trapped by nucleophiles to give substitution product or may deprotonate to yield a small amount of alkene.
If the leaving group present eliminates along with a proton in absence of any strong nucleophile the double bond formation might occur. Such reactions are known as two-step unimolecuar elimination, abbreviated as
(c)
Interpretation: The mechanism II should be identified.
Concept introduction: Carbocations generated from alkyl halides have two fates; they can be either trapped by nucleophiles to give substitution product or may deprotonate to yield a small amount of alkene.
If the leaving group present eliminates along with a proton in absence of any strong nucleophile the double bond formation might occur. Such reactions are known as two-step unimolecuar elimination, abbreviated as
(d)
Interpretation: The approximate molar concentration of sodium methoxide that would cause the two mechanisms to proceed at same rate mechanism should be identified.
Concept introduction: Carbocations generated from alkyl halides have two fates; they can be either trapped by nucleophiles to give substitution product or may deprotonate to yield a small amount of alkene.
If the leaving group present eliminates along with a proton in absence of any strong nucleophile the double bond formation might occur. Such reactions are known as two-step unimolecuar elimination, abbreviated as
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: Structure and Function
- (a) Select all of the correct statements about reaction rates from the choices below. The lower the rate of a reaction the longer it takes to reach completion.Reaction rate constants increase with increasing temperature.Catalysts increase reaction rates.Fast reactions can be slowed down by lowering the temperature.Slow reactions can be speeded up by raising the temperature.The rate of a reaction is the same as the rate constant of the reaction.Reaction rate constants decrease with increasing temperature.arrow_forward(a) Select all of the correct statements about reaction rates from the choices below. 1.The lower the rate of a reaction the longer it takes to reach completion.2 The rate of a reaction is independent of temperature.3 Slow reactions can be speeded up by raising the temperature.4. Reaction rates increase with increasing temperature.5. Solid catalysts do not affect reaction rates.6. Reactions involving very unstable combinations of chemicals have large rate constants.7. A balanced chemical reaction is necessary to relatearrow_forward(а) (b) (c) (d) all react at same ratearrow_forward
- Which of the following reactions will be fastest? (a) Br: (c) Br: H S. CF3 (b) Br: (d) Br: CH3 OHarrow_forwardThe mechanism for the reaction of CH3OH andHBr is believed to involve two steps. The overallreaction is exothermic.Step 1: Fast, endothermicCH3OH + H+ uv CH3OH2+Step 2: SlowCH3OH2+ + Br− n CH3Br + H2O(a) Write an equation for the overall reaction.(b) Draw a reaction coordinate diagram for thisreaction.(c) Show that the rate law for this reaction isRate = k[CH3OH][H+][Br−].arrow_forwardThe following reaction proceeds at a rate such that 4 mole of AA is consumed per minute. Given this, how many moles of CC are produced per minute? 2A+2B→4Carrow_forward
- Consider the energy diagram shown below when answer questions G B C D E Progress of Reaction (a) The rate-determining step for the reaction is (А -> С, С -> Е, or E -> G) (b) The fastest step in this reaction is (А > С, С —> Е, or E > G) (c) The transition states for this reaction are (d) This reaction is clearly (exergonic, endergonic) (e) Which is more stable, intermediate C or product E? (f) Based on the Hammond Postulate, transition state F is more similar in structure to which species, E or G? Free Energyarrow_forwardFor the reaction shown, the rate of reaction when X=Cl is about the same as that when X=Br. Based on this information, which statement represents a valid deduction concerning the mechanism of this reaction? OCH2CH3 O2N NO2 NO2 (A) This is a one-step displacement reaction (B) The reaction proceeds via a benzyne intermediate (C) The C-X bond is broken in the rate-determining step (D) The C-X bond is NOT broken in the rate-determining step O2N OCH,CH3 NO2 NO2arrow_forwarda) Balance the reaction formula for the following reaction: CH3COOH + 2C5H2O → 2C5H11OCOCH3 + H2O b) Draw your own reaction mechanism for the reaction.arrow_forward
- When the following reactions are carried out under the same conditions, the rate constant for the first reaction (kH) is found to be 7 times greater than the rate constant for the second reaction (kD). What does that tell you about the mechanism of the reaction? (Hint: a C- D bond is 1.2 kcal/mol stronger than a C—H bond.)arrow_forward(1) The activation energy for the gas phase isomerization of methyl cis-cinnamate is 174 kJ.cis-C6H5CH=CHCOOCH3trans-C6H5CH=CHCOOCH3The rate constant at 621 K is 8.10×10-5 /s. The rate constant will be 8.42×10-4 /s at_____ K. (1b) The activation energy for the gas phase isomerization of methyl cis-cinnamate is 174 kJ.cis-C6H5CH=CHCOOCH3trans-C6H5CH=CHCOOCH3The rate constant at 654 K is 4.57×10-4 /s. The rate constant will be /s at _______ 695 K.arrow_forwardWrite the mechanism for the following reaction: `Br ELOH Write the mechanism and predict if the reaction above be faster or slower than the following reaction? Explain. Br ELOHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





