
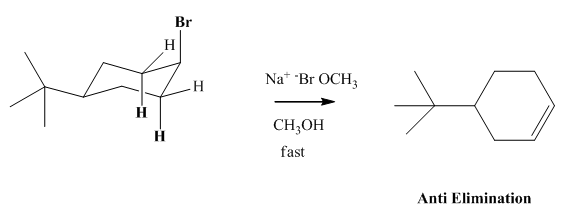
(a)
Interpretation: The reason behind rate of bimolecular elimination in
Concept introduction: Experimental evidence for
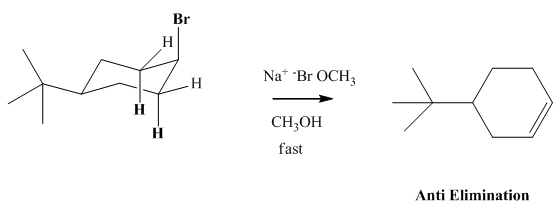
(b)
Interpretation: The formation of only specific elimination product should be explained.
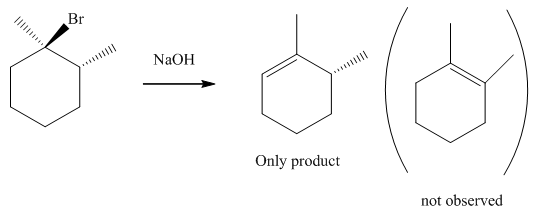
Concept introduction: Experimental evidence for
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: Structure and Function
- (i) Decide which the following compounds will react faster in an E2 reaction; trans-1-bromo-2-isopropylcyclohexane or cis-1-bromo-2-isopropylcyclohexane. (ii) Explain your answer.arrow_forwardPredict the elimination products of the following reactions, and label the major products.(a) cis-1-bromo-2-methylcyclohexane + NaOCH3 in CH3OHarrow_forward(a) When cis-1-bromo-2-methylcyclohexane undergoes an E2 reaction, two products (cycloalkenes) are formed. What are these two cycloalkenes, and which would you expect to be the major product? Write conformational structures showing how each is formed. (b) When rans-1-bromo-2-methylcyclohexane reacts in an E2 reaction, only one cyclo- alkene is formed. What is this product? Write conformational structures showing why it is the only product.arrow_forward
- Predict the major products of the following reactions, including stereochemistry.(a) cyclohexene + KMnO4>H2O (cold, dilute)(b) cyclohexene + peroxyacetic acid in waterarrow_forwardThe treatment of (CH3)2C=CHCH2Br with H2O forms B (molecular formulaC5H10O) as one of the products. Determine the structure of B from its 1H NMR and IR spectra.arrow_forwardRank the compounds in each group in order of increasing reactivity in electrophilic aromatic substitution: (a) C6H6, C6H5Cl, C6H5CHO, C6H5OCH3; (b) C6H5CH3, C6H5NH2, C6H5CH2NH2, C6H5CONH2.arrow_forward
- The treatment of (CH3)2C=CHCH2Br with H2O forms B (molecular formula C5H10O) as one of the products. Determine the structure of B from its 1H NMR and IR spectra.arrow_forwardThere are a variety of isometric 1,2,3,4,5,6-hexachlorocyclohexanes, but one of them does not react with CH3CH2O- to give an elimation product. Provide the structures of this compound and briefly explain why it does not reactarrow_forward2. (a) Give the product of the following reaction: Br t-BUOK (b) Using the FMOS of the reacting partners explain why this reaction is expected to occur simply on heating, in the absence of light. (c) Specify the preferred stereoisomer and explain why it is preferred.arrow_forward
- Friedel–Crafts alkylation of benzene with (R)-2-chlorobutane and AlCl3 affords sec-butylbenzene. Would you expect the product to exhibit optical activity? Explain, with reference to the mechanism.arrow_forwardReaction of this bicycloalkene with bromine in carbon tetrachloride gives a trans dibro- mide. In both (a) and (b), the bromine atoms are trans to each other. However, only one of these products is formed. CH3 CH3 CH3 Br Br CH,Cl, + Br2 or Br Br (a) (b) Which trans dibromide is formed? How do you account for the fact that it is formed to the exclusion of the other trans dibromide?arrow_forward(c) The E2 elimination of bromoalkane 8 produces a range of alkenes as shown below, answer ALL parts (i) to (iii) Br 8 Ph NaOMe Ph 9 + FOUR more alkenes (i) Identify the structure of the four other possible alkene products. (ii) Assign the stereochemistry (E/Z) for all FIVE alkenes and explain which is likely to be the major product. (iii) With an explanation identify which stereoisomer of 8 would give alkene 9?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





