
Concept explainers
(a)
Interpretation:The hashed and wedged formulas should be converted to condensed form.
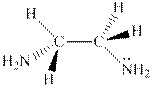
Concept introduction:There are three ways of representation of molecular structures. The hashed and wedged formulas have three types of bonds. The bond above the plane is represented by bold hash while bonds behind the plane are represented by wedge.The bonds in plane are represented by normal bonds.
Bond-line formula depicts the zig-zag framework of carbon chain with attached substituents. The terminal position represents a methyl while the non-terminal is for methylene group. For instance, the propane is written in this notation as follows:
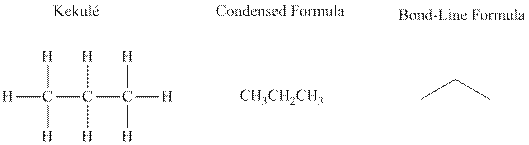
(b)
Interpretation: The hashed and wedged formulas should be converted to condensed form.
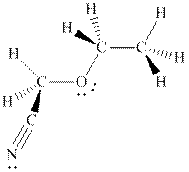
Concept introduction: There are three ways of representation of molecular structures. The hashed and wedged formulas have three types of bonds. The bond above the plane is represented by bold hash while bonds behind the plane are represented by wedge.The bonds in plane are represented by normal bonds.
Bond-line formula depicts the zig-zag framework of carbon chain with attached substituents. The terminal position represents a methyl while the non-terminal is for methylene group. For instance, the propane is written in this notation as follows:
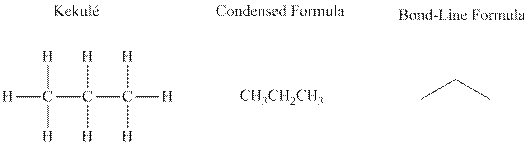
(c)
Interpretation: The hashed and wedged formulas should be converted to condensed form.
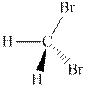
Concept introduction: There are three ways of representation of molecular structures. The hashed and wedged formulas have three types of bonds. The bond above the plane is represented by bold hash while bonds behind the plane are represented by wedge.The bonds in plane are represented by normal bonds.
Bond-line formula depicts the zig-zag framework of carbon chain with attached substituents. The terminal position represents a methyl while the non-terminal is for methylene group. For instance, the propane is written in this notation as follows:
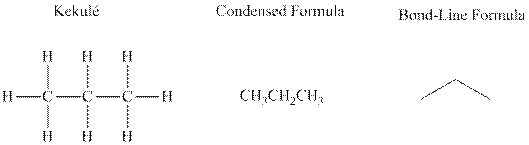
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Organic Chemistry: Structure and Function
- Apply the significant figure rule to the final answer. Example attached and dig figures rulesarrow_forwardWrite the condensed structure of a compound that contains only carbon and hydrogen atoms and that has. please help with al partsarrow_forwardADVANCED MATERIAL Interpreting condensed chemical structures Use this condensed chemical structure to complete the table below. CH,= CH- CH= CH, The condensed chemical structure of 1,3-butadiene Some facts about the 1,3-butadiene molecule: number of carbon-carbon single (C - C) bonds: number of carbon-hydrogen single (C - H) bonds: number of carbon-carbon double (C = C) bonds: te Roserved.arrow_forward
- Please don't provide handwritten solution ....arrow_forwardDoxorubicin, shown here, is an important chemotherapy drug used to treat avariety of cancers, including bladder cancer, breast cancer, and certain forms of leukemia. Doxorubicin works by binding to DNA in such a way that a portion of it penetrates the DNA double helix— a process called intercalation. During transcription— the process that forms RNA— portions of the DNA strands are temporarily separated for the base sequence to be read and then are reconnected. With bound doxorubicin, however, the double helix does not reform properly after the strands are separated, which disrupts replication— the process that forms an identical copy of DNA. Which portion of doxorubicin do you think intercalates into the DNA double helix, and why do you think it has little difficulty doing so?arrow_forwardDraw the condensed structure of an isomer of this molecule: CH3-CH2 -CH2 CH3 Click anywhere to draw the first atom of your structure.arrow_forward
- IV. Fill up the numbered spaces (only) in the table below. Type your answer for the functional group. For the chemical formula, draw it manually, scan copy or shot a picture and paste in the answer sheets. Functional Structural Formula (SF) Condensed SF Condensed Formula Bond-line or Line-angle Group Formula 1 2 CH3 CH3-CH2-C=C-C-CHs CH3 4arrow_forwardQuestion: Consider a hypothetical molecule, X₄Y₃, composed of four atoms of element X and three atoms of element Y. The molecule is in a gaseous state. a) According to the octet rule, which elements are likely to form multiple bonds in this molecule? Explain your reasoning. b) Calculate the total number of valence electrons in X₄Y₃. c) Based on the total number of valence electrons, determine the formal charge on each atom in X₄Y₃.arrow_forwardWrite the dash formula of the following compounds. Expand the following condensed formulas so as to show all the bonds and unshared electron pairs.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





