
Concept explainers
Interpretation:Compounds
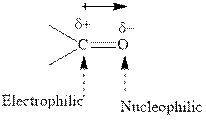
Concept introduction:The difference in electronegativity gives rise to partial positive and negative charges on two atoms. The extent of polarity is quantitatively expressed as dipole moment. It is quantitatively defined as product of charges of two bonded atoms and the separation between them. The electronegativity difference decides the direction of dipole moment. It is indicated by an arrow head from positive end towards negative end. For example,the carbonyl bond is polar with partial postive charge on carbon and partial negative charge on oxygen as illustrated below.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Organic Chemistry: Structure and Function
- 13-27 Define autoxidation.arrow_forwardFormaldehyde, H2C=O, is known to all biologists because of its usefulness as a tissue preservative. When pure, formaldehyde trimerizes to give trioxane, C3H6O3, which, surprisingly enough, has no carbonyl groups. Only one monobromo derivative (C3H5BrO3) of trioxane is possible. Propose a structure for trioxane.arrow_forwardEistrogens are female sex hormones, the most potent of which is B-estradiol. OH H,C но B-Estradiol In recent years, chemists have focused on designing and synthesizing molecules that bind to estrogen receptors. One target of this research has been nonsteroidal estrogen antagonists, compounds that interact with estrogen receptors and block the effects of both endogenous and exogenous estrogens. A feature common to one type of nonste- roidal estrogen antagonist is the presence of a 1,2-diphenylethylene with one of the benzene rings bearing a dialkylaminoethoxyl substituent. The first nonsteroidal estrogen antagonist of this type to achieve clinical importance was tamoxifen, now an important drug in the treatment of breast cancer. Tamoxifen has the Z configuration shown here. но NMe, NMeg ? B NMeg ? "NMe, ОН Tamoxifen Propose reagents for the conversion of A to tamoxifen. Note: The final step in this synthesis gives a mixture of E and Z isomers.arrow_forward
- Estrogens are female sex hormones, the most potent of which is B-estradiol. OH AYA Но B-Estradiol In recent years, chemists have focused on designing and synthesizing molecules that bind to estrogen receptors. One target of this research has been nonsteroidal estrogen antagonists, compounds that interact with estrogen receptors and block the effects of both endogenous and exogenous estrogens. A feature common to one type of nonste- roidal estrogen antagonist is the presence of a 1,2-diphenylethylene with one of the benzene rings bearing a dialkylaminoethoxyl substituent. The first nonsteroidal estrogen antagonist of this type to achieve clinical importance was tamoxifen, now an important drug in the treatment of breast cancer. Tamoxifen has the Z configuration shown here. NMeg A B NMeg ? ОН Tamoxifen Propose reagents for the conversion of A to tamoxifen. Note: The final step in this synthesis gives a mixture of E and Z isomers.arrow_forwardIn an advanced analytical chemistry lab, a team analyzing a compound 'Q' known to be a structural isomer of octane (C8H18). To determine the specific structure of 'Q', a series of spectroscopic analyses are performed. The sequence of the analysis involves: Infrared (IR) spectroscopy, which indicates the absence of functional groups like alcohols, ketones, and carboxylic acids. Nuclear Magnetic Resonance (NMR) spectroscopy, showing signals indicative of only methyl and methylene groups, with no evidence of methine (CH) or quaternary carbon environments. Mass spectrometry (MS), revealing a fragmentation pattern consistent with branched alkane structures. Based on this sequence of analyses, what is the most likely structure of compound 'Q'? Options: A. 2,2,4- Trimethylpentane B. n-Octane C. 2-Methylheptane D. 3-Ethylhexane Don't use chatgpt please provide valuable answerarrow_forwardDo not give handwriting solution.arrow_forward
- Ester formation and ester hydrolysis are exactly the same reaction only written in reverse. General reaction of ester formation: H*, heat R—с—он + Н—о—R' R—с—о-R' + H,O carboxylic acid alcohol carboxylic or phenol ester General reaction of ester hydrolysis: || R—с—OR' + H—оН R—с—оН +R—ОH ester carboxylic acid alcohol or phenol What determines which direction the reaction proceeds and what actually forms? o the boiling point of the carboxylic acid o the presence (or absence) of heat as well as the concentration of reactants and products o the molecular weight of reactants and products o the presence (or absence) of heat as well as the catalystarrow_forwardIdentify the functional group/s of the following organic compounds `N ОН Соcaine A°-Tetrahydrocannabinol (THC) Lysergide (lysergic acid diethyl amide, or LSD) `NH НО. HN + H3N ОН Aspartame (NutraSweet) Theobromine Caffeine Azidothymidine (AZT) ОН F3C. 'N H2 Penicillin G НО Propofol Fluoxetine hydrochloride (Prozac) HO Na+ Dextromethamphetamine Naproxen sodium (Aleve) Acetylsalicylic acid (Aspirin)arrow_forwardCore 2 (a) The structure of limonene is shown below. CH₂ CH₂ CH₂ CH CH₂ (i) What is the molecular formula of limonene? **********‒‒‒‒‒‒‒‒‒‒‒‒‒‒‒ (II) Some limonene was added to a few drops of aqueous bromine. What colour change would you see in the aqueous bromine? ************ (iii) What feature of a limonene molecule is responsible for this colour change? (iv) Name the two substances formed when limonene is burnt in an excess of oxygen.arrow_forward
- Give at least 5 examples of biological compounds having an ether functional group and identify the biochemical importance of each compound.arrow_forwardN,N-Dimethyltryptamine (DMT) is a powerful hallucinogenic that is being trialled (in low doses) to treat depression, mania and anxiety disorders. In the clinical trials where the researchers aim to test this compound on people, it is found that the drug (drawn below) is poorly absorbed from the gut into the blood plasma. Further, the drug has poor solubility in the blood as it is very non-polar (over-all). Name a reaction that could assist with solubilising DMT in the blood. O Inject DMT directly into the blood intravenously, to avoid uptake via the gut Take DMT via the respiratory route (smoke or inhale it) to increase uptake into the blood Dissolve DMT in an oil immersion to increase its uptake in the gut O React with HCI to afford the hydrochloride salt of each basic amine, thus increasing water solubility ZI Ooooarrow_forwardThe following figure shows a typical phospholipid. Suppose this molecule was incorporated into a cell membrane. Which letter indicates the portion of the molecule that would be exposed to the aqueous environment?arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





