
Concept explainers
(a)
Interpretation:Theoctet resonance structures needs to be drawn and the strongest resonance contributor needs to be indicated.
Concept Introduction:VSEPR theory stands as Valence Shell Electron Pair Repulsion Theory. It helps to predict the molecular shape or geometry of the molecule with the help of the number of bond pair or lone pair present in it. According to VSEPR theory, the presence of lone pair on the central atom of molecule causes deviation from standard molecular geometry.
The resonance is the phenomenon in which if all the properties cannot explain by one structure, it can be shown in two or more structures by the shifting of pi bonds or lone pair but there is no change in sigma bond and position of atoms.
(b)
Interpretation: The octet resonance structures needs to be drawn and the strongest resonance contributor needs to be indicated.
Concept Introduction:VSEPR theory stands as Valence Shell Electron Pair Repulsion Theory. It helps to predict the molecular shape or geometry of the molecule with the help of the number of bond pair or lone pair present in it. According to VSEPR theory, the presence of lone pair on the central atom of molecule causes deviation from standard molecular geometry.
The resonance is the phenomenon in which if all the properties cannot explain by one structure, it can be shown in two or more structures by the shifting of pi bonds or lone pair but there is no change in sigma bond and position of atoms.
(c)
Interpretation: The octet resonance structures needs to be drawn and the strongest resonance contributor needs to be indicated.
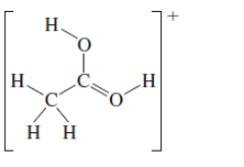
Concept Introduction:VSEPR theory stands as Valence Shell Electron Pair Repulsion Theory. It helps to predict the molecular shape or geometry of the molecule with the help of the number of bond pair or lone pair present in it. According to VSEPR theory, the presence of lone pair on the central atom of molecule causes deviation from standard molecular geometry.
The resonance is the phenomenon in which if all the properties cannot explain by one structure, it can be shown in two or more structures by the shifting of pi bonds or lone pair but there is no change in sigma bond and position of atoms.
(d)
Interpretation: The octet resonance structures needs to be drawn and the strongest resonance contributor needs to be indicated.
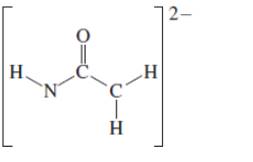
Concept Introduction:VSEPR theory stands as Valence Shell Electron Pair Repulsion Theory. It helps to predict the molecular shape or geometry of the molecule with the help of the number of bond pair or lone pair present in it. According to VSEPR theory, the presence of lone pair on the central atom of molecule causes deviation from standard molecular geometry.
The resonance is the phenomenon in which if all the properties cannot explain by one structure, it can be shown in two or more structures by the shifting of pi bonds or lone pair but there is no change in sigma bond and position of atoms.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Organic Chemistry: Structure and Function
- You will not find “hydroxide” in the stockroom, but you will find sodium hydroxide (NaOH) andpotassium hydroxide (KOH). Lithium hydroxide (LiOH) is expensive and used in spacecraft airfilters since hydroxide reacts with carbon dioxide, and lithium is lighter than sodium or potassium.Cesium and francium hydroxides are very expensive and little used. Is this information consistentwith your answer to the previous question?arrow_forwardWhich compound has the greatest ionic character? O RbF O LiF O NaF O CSF Question 2 Which of the following compound has the central atom with +1 formal charge? O PCI6 O XEF3* O IF5 O AICIAarrow_forwardWhich is the strongest bond energy? Cl(g) ΔH°f, = +121.3 kJ mol–1 CCl4(g) ΔH°f, = –95.98 kJ mol–1 HCl(g) ΔH°f, = –92.3 kJ mol–1 Question 8 options: Cl–Cl in Cl2 H–Cl in HCl C–Cl in CCl4arrow_forward
- Write Lewis Structure and Determine the Formal Charge of H C N H N C Which is the best way to represent hydrocyanic acid? Why?arrow_forwardAdd formal charges to each resonance form of HCNO. Resonance structure A Incorrect H-CIN= 0: Resonance structure B Incorrect HC=N:arrow_forwardEnter your answer in the provided box. Use bond energies to calculate the heat of reaction: H O + 2NH H C-N -0 |C=O C=O (in CO₂) O-H N-H Bond Enthalpy 305 358 745 799 467 391 kJ mol H-N- C H -N—H + H-O-H Harrow_forward
- Which pair(s) of structures below are resonance contributors to the same resonance hybrid? II II I only Il only Il and III only I and III only I, II, and III O 0 0 O Oarrow_forwardWhat is the formal charge on N in this ion? -2 O-1 +1 O +2arrow_forwardHow many of the following charged structures have two (or more) resonance contributors of approximately equivalent energy? N: H2N 1 2 3 4arrow_forward
- Explain why the following reaction is endothermic based on your knowledge of both bond enthalpy and endothermic reaction profile. Draw the endothermic reaction profile to help. 2NaHCO3 = Na2CO3 + H2O + CO2 DH = positivearrow_forwardCH, Use bond dissociation enthalpies to estimate the enthalpy change in this reaction. AH (kJ/mol) Bond H-O C-C H-C 010 C- 010 + H₂O(g) → CH₂CH(OH)CH₂(g) Enthalpy change 44 Submit Substance 463 610 413 346 358 bCalculate the enthalpy change for this reaction from enthalpies of formation. AƒH (kJ/mol) la 272.8arrow_forwardThe carbonate anion, CO32- , is a resonance hybrid. Draw all of the important resonance structures for this molecule. If an atom has a nonzero formal charge, be sure the formal charge is shown clearly in the structure. Use the resonance structures to calculate the average formal charge on each O atom (which are all equivalent in the "true" structure). [Note: all of the important contributing resonance structures have octets around each atom that desires an octet.]arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning



