
Concept explainers
a.
Interpretation:The following Kekulé formula should be represented in its condensed formula:
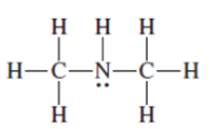
Concept introduction: While writing the condensed formula of the compound, all the atoms in the compound are written a line of text and the vertical bonds and lone pairs are omitted.
b.
Interpretation: The following Kekulé formula should be represented in its condensed formula:
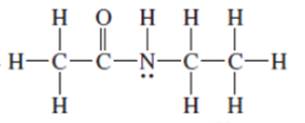
Concept introduction: While writing the condensed formula of the compound, all the atoms in the compound are written a line of text and the vertical bonds and lone pairs are omitted.
c.
Interpretation: The following Kekulé formula should be represented in its condensed formula:
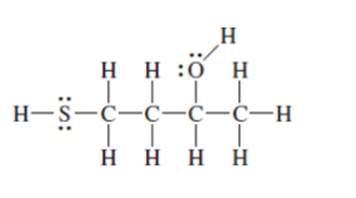
Concept introduction: While writing the condensed formula of the compound, all the atoms in the compound are written a line of text and the vertical bonds and lone pairs are omitted.
d.
Interpretation: The following Kekulé formula should be represented in its condensed formula:
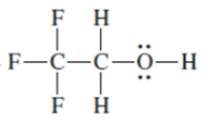
Concept introduction: While writing the condensed formula of the compound, all the atoms in the compound are written a line of text and the vertical bonds and lone pairs are omitted.
e.
Interpretation: The following Kekulé formula should be represented in its condensed formula:
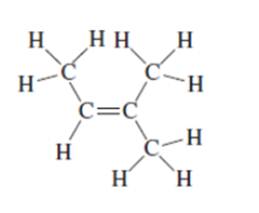
Concept introduction: While writing the condensed formula of the compound, all the atoms in the compound are written a line of text and the vertical bonds and lone pairs are omitted.
f.
Interpretation: The following Kekulé formula should be represented in its condensed formula:
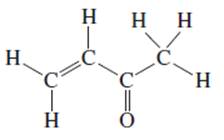
Concept introduction: While writing the condensed formula of the compound, all the atoms in the compound are written a line of text and the vertical bonds and lone pairs are omitted.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Organic Chemistry: Structure and Function
- Complete the following table for TNT (trinitrotoluene), C7H5(NO2)3.arrow_forward4 2-15. The plate is subjected to the forces acting on members A and B. If 0 = 601 determine the magnitude of the resultant of these forces and its direction measured clockwise from the positive x axis.arrow_forwardH2SO3 is: O sodium hydroxide O sulfurous acid O hydrochloric acid O sulfuric acidarrow_forward
- Sodium citrate (Na3C6H5O7) is an ionic compound. Draw a diagram or use 2-3 sentences explain the charges on the different ions in this compound.arrow_forwardGive the systematic name of the compound. OH HC CH3 CH3 он The name of the compound isarrow_forwardWhich of the formulas must be molecular formulas? PO H2O2 C5H9 C2H4O2 C3H4O3arrow_forward
- Complete the columns in the table: formula: H3PO4(aq) name: calcium nitrate hydrogen sulfide substance type:arrow_forwardis the chemical formula, C2OH2N2O2, how would you write 3 molecules of this compoundarrow_forwardWrite the meaning of formula unit How is it determined? Calculate the formula unit mass of a compound, Na,S,O,. (Given, atomic mass of Na-23 u; S-32 u; 0=16 u)arrow_forward
- Name of Compound Formula of Compound Ammonium nitrate Iron(III) carbonate Copper (I) sulphide Ca3(PO4)2 FeSO4arrow_forwardPredict whether the following compounds are ionic or molecular:(a) KI, the compound used as a source of iodine in table salt(b) H2O2, the bleach and disinfectant hydrogen peroxide(c) CHCl3, the anesthetic chloroform(d) Li2CO3, a source of lithium in antidepressantsarrow_forwardWhat is the molecular formula of the compound (which contains only carbon, hydrogen, and oxygen)?arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





