
Concept explainers
(a)
Interpretation:The Kekulè (straight line) notation for the given bond line formula needs to be drawn.
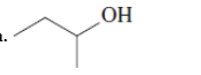
Concept Introduction: The chemical compounds can be shown in different structural formulae. Condense formula, wedge-dashed formula and bond-line formula are some common ways to show the structural of chemical compounds.
In wedge-dashed line formula, the groups or atoms bonded to each C atoms must be shown with dashed and wedges. Here wedge is the solid line that represents the bond in plane of the surface whereas dash line represents the line extended back behind the surface.
In the bond line formula, each C is shown as dot and the each dot is connected with another dot with line. Excluding C and H, all other elements of the compound like N, S, O must be indicted with their atomic symbols. In Kekulè (straight line) notation all atoms and
(b)
Interpretation: The Kekulè (straight line) notation for the given bond line formula needs to be drawn.
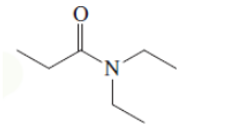
Concept Introduction: The chemical compounds can be shown in different structural formulae. Condense formula, wedge-dashed formula and bond-line formula are some common ways to show the structural of chemical compounds.
In wedge-dashed line formula, the groups or atoms bonded to each C atoms must be shown with dashed and wedges. Here wedge is the solid line that represents the bond in plane of the surface whereas dash line represents the line extended back behind the surface.
In the bond line formula, each C is shown as dot and the each dot is connected with another dot with line. Excluding C and H, all other elements of the compound like N, S, O must be indicted with their atomic symbols. In Kekulè (straight line) notation all atoms and chemical bonds must be shown with any symbol of lone pairs on atoms if any.
(c)
Interpretation: The Kekulè (straight line) notation for the given bond line formula needs to be drawn.
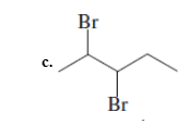
Concept Introduction: The chemical compounds can be shown in different structural formulae. Condense formula, wedge-dashed formula and bond-line formula are some common ways to show the structural of chemical compounds.
In wedge-dashed line formula, the groups or atoms bonded to each C atoms must be shown with dashed and wedges. Here wedge is the solid line that represents the bond in plane of the surface whereas dash line represents the line extended back behind the surface.
In the bond line formula, each C is shown as dot and the each dot is connected with another dot with line. Excluding C and H, all other elements of the compound like N, S, O must be indicted with their atomic symbols. In Kekulè (straight line) notation all atoms and chemical bonds must be shown with any symbol of lone pairs on atoms if any.
(d)
Interpretation: The Kekulè (straight line) notation for the given bond line formula needs to be drawn.
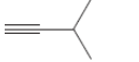
Concept Introduction: The chemical compounds can be shown in different structural formulae. Condense formula, wedge-dashed formula and bond-line formula are some common ways to show the structural of chemical compounds.
In wedge-dashed line formula, the groups or atoms bonded to each C atoms must be shown with dashed and wedges. Here wedge is the solid line that represents the bond in plane of the surface whereas dash line represents the line extended back behind the surface.
In the bond line formula, each C is shown as dot and the each dot is connected with another dot with line. Excluding C and H, all other elements of the compound like N, S, O must be indicted with their atomic symbols. In Kekulè (straight line) notation all atoms and chemical bonds must be shown with any symbol of lone pairs on atoms if any.
(e)
Interpretation: The Kekulè (straight line) notation for the given bond line formula needs to be drawn.

Concept Introduction: The chemical compounds can be shown in different structural formulae. Condense formula, wedge-dashed formula and bond-line formula are some common ways to show the structural of chemical compounds.
In wedge-dashed line formula, the groups or atoms bonded to each C atoms must be shown with dashed and wedges. Here wedge is the solid line that represents the bond in plane of the surface whereas dash line represents the line extended back behind the surface.
In the bond line formula, each C is shown as dot and the each dot is connected with another dot with line. Excluding C and H, all other elements of the compound like N, S, O must be indicted with their atomic symbols. In Kekulè (straight line) notation all atoms and chemical bonds must be shown with any symbol of lone pairs on atoms if any.
(f)
Interpretation: The Kekulè (straight line) notation for the given bond line formula needs to be drawn.
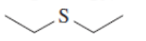
Concept Introduction: The chemical compounds can be shown in different structural formulae. Condense formula, wedge-dashed formula and bond-line formula are some common ways to show the structural of chemical compounds.
In wedge-dashed line formula, the groups or atoms bonded to each C atoms must be shown with dashed and wedges. Here wedge is the solid line that represents the bond in plane of the surface whereas dash line represents the line extended back behind the surface.
In the bond line formula, each C is shown as dot and the each dot is connected with another dot with line. Excluding C and H, all other elements of the compound like N, S, O must be indicted with their atomic symbols. In Kekulè (straight line) notation all atoms and chemical bonds must be shown with any symbol of lone pairs on atoms if any.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Organic Chemistry: Structure and Function
- Diazomethane (CH2N2) is an important reagent for the methylation of some organic molecules. Complete Parts 1 and 2 below about this unique reagent. Draw the Lewis structure of diazomethane (CH2N2) that contains a formal charge on carbon and nitrogen. Be sure to include all lone pairs of electrons and formal charges.arrow_forwardConvert each of the following molecular models into a skeletal structure, and give the formula of each. Only the connections between atoms are shown; multiple bonds are not indicated (gray = C, red = O, blue = N, ivory = H).arrow_forward1. For each of the following compounds and ions, draw the complete Lewis structure. All formal charges should be on the atoms that possess that formal charge. CH4 H3CBr CH3CH₂OH H₂S H₂SO4 (sulfuric acid) H³0+ CH3* 2. Convert the following condensed formulas into bond-line structures, making sure to draw in zig-zag formation. CH3CH₂CH₂CH₂CH₂CH₂CH₂CH3 FCH₂CH₂I H₂C=CHCH₂OH (CH3)2CHCOOHarrow_forward
- Determine a molecular formula, e.g. CH4, from the line structure below. Specify elements in the following order: C, H, others(in alphabetical order). Example: C4H7ClOS Molecular formulaarrow_forwardUnshared, or lone, electron pairs play an important role in determining the chermical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. The number of unshared pairs at atom a is The number of unshared pairs at atom b is HyC CH The number of unshared pairs at atom e is The number of unshared pairs at atom a is The number of unshared pairs at atom b is HC CH The number of unshared pairs at atom e isarrow_forwardMethyl isocyanate, CH3 -N= C = O, is used in the industrial synthesis of a type of pesticide and herbicide known as a carbamate. As a historical note, an industrial accident in Bhopal, India, in 1984 resulted in leakage of an unknown quantity of this chemical into the air. An estimated 200,000 people were exposed to its vapors, and over 2000 of these people died. Q.) Write a Lewis structure for methyl isocyanate and predict its bond angles. What is the hybridization of its carbonyl carbon? Of its nitrogen atom?arrow_forward
- Linoleic acid (below) is an essential fatty acid found in many vegetable oils, such as soy, peanut, and cottonseed. A key structural feature of the molecule is the cis orientation around its two double bonds, where R1 and R2 represent two different groups that form the rest of the molecule. R, CH2 `H H' (a) How many different compounds are possible, changing only the cis/trans arrangements around these two double bonds? (b) How many are possible for a similar compound with three double bonds? R3. .CH2 R4 `H H `H H'arrow_forwardConvert each of the following molecular models into a skeletal structure, and give the formula of each. Only the connections between atoms are shown; multiple bonds are not indicated (gray=C, red =O, blue =N, ivory = H)arrow_forwardLinoleic acid is an essential fatty acid found in many veg-etable oils, such as soy, peanut, and cottonseed. A key structural feature of the molecule is the c is orientation around its two dou-ble bonds, where R1and R2 represent two different groups thatform the rest of the molecule. (a) How many different compounds are possible, changing onlythe cis-trans arrangements around these two double bonds?(b) How many are possible for a similar compound with three double bonds?arrow_forward
- What is the molecular formula for the following bond-line structures? H,arrow_forwardDraw the shapes of the following molecules and ions in 3-dimension. Show clearly any lone pairs of electrons on the central atom, state the number of bond pairs and lone pairs of electrons on the central atom and name the shape of the molecule or ion. (a) CH3+, methyl carbocation (b) HOBr, bromic (I) acid (c) NCl3, nitrogen trichloride Provide everything stated in the instructions for each compound.arrow_forward2. Now write the condensed formulas for the following bond-line structures. la он Н HO.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
