
Concept explainers
(a)
Interpretation:Two resonance forms for
Concept introduction:When a certain Lewis structure proposed is not able to describe all the experimentally observed parameters of a molecule two or more Lewis structures that differ only in the location of multiple bonds are drawn. These are known as resonance structures. The actual molecule is essentially represented as the hybrid of each of such resonance structures. For instance, a molecule such as ozone
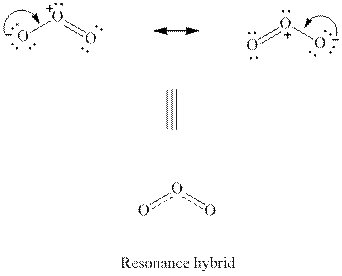
(b)
Interpretation:Out of the two resonanceformsof
Concept introduction: When a certain Lewis structure proposed is not able to describe all the experimentally observed parameters of a molecule two or more Lewis structures that differ only in the location of multiple bonds are drawn. These are known as resonance structures. The actual molecule is essentially represented as the hybrid of each of such resonance structures. For instance, a molecule such as ozone
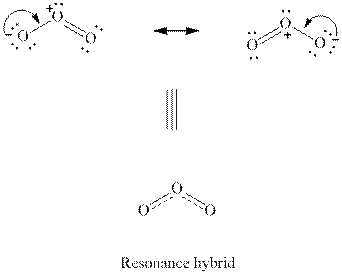
(c)
Interpretation:The manner the relative importance of the resonance forms
Concept introduction: When a certain Lewis structure proposed is not able to describe all the experimentally observed parameters of a molecule two or more Lewis structures that differ only in the location of multiple bonds are drawn. These are known as resonance structures. The actual molecule is essentially represented as the hybrid of each of such resonance structures. For instance, a molecule such as ozone
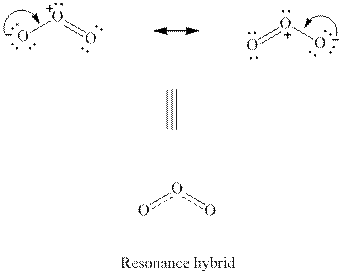
(d)
Interpretation: The hybridization of nitrogen in
Concept introduction:The table that relates the steric number with hybridization is as follows:
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Organic Chemistry: Structure and Function
- 3-31 Why does electronegativity generally increase going from left to right across a row of the Periodic Table?arrow_forwardDraw the Lewis structure of BH4- and use your Lewis structure to help fill in the missing numbers. Hint: you must put a number in each box. If the answer is zero, you must enter "0". The Lewis structure has: lone pairs = single bonds = double bonds = triple bonds = atoms with a positive formal charge = atoms with a negative formal charge = Is this structure stabilised by resonance? (Enter either yes or no below - no other words).arrow_forwardНС (5) HOCH, CH,CH,CH,OH ZnCl2arrow_forward
- Is there an OH group present? Are there any C(sp2 )–H bonds? How about a carbon-carbon triple bond (alkyne) or a carbon-nitrogen triple bond (nitrile)? Is there a C=O?arrow_forwardUsing the symbols 8- and &+, indicate the direction of polarity, if any, in each covalent bond. (а) С—СІ (b) S-H (c) C-S (d) Р—Нarrow_forward2RO; (aq) + 12 H* (aq) + 3 M (s) = RO (g)+3M²* (aq) + 6 H2O (1) = 3.86 V. R: 5 valence e-, electronegativity of R = 3.3 M: 2 valence e-, electronegativity of M = 0.5 O and H represent Oxygen and Hydrogen (the known elements, with their known properties) All atoms follow the octet rule; except for Hydrogen which follows the duet rule. b) Calculate the new cell potential if concentrations of all the reactants is deceased to 0.5 M, and the concentrations of all the products is increased to 0.5 M. Assume 6 mol electrons transferred in the reaction.arrow_forward
- 1₁ 9) CNN B G O Bond H-O C=C H-C C-C C-O This question has multiple parts. Work all the parts to get the Enthalpy change COWLV2... 2-Propanol can be made by the reaction of propene and water: CH3-HC=CH₂(g) + H₂O(g) → CH3 CH(OH)CH3(g) a Use bond dissociation enthalpies to estimate the enthalpy change in this reaction. ΔΗ (kJ/mol) Substance 463 610 413 346 358 CH3 CH(OH)CH3(g) Enthalpy change = b Calculate the enthalpy change for this reaction from enthalpies of formation. Af H (kJ/mol) H₂O(g) CH3-H₂C=CH₂(g) KJ * prod03-cnow-owl.cengagenow.c -272.8 -241.83 20.42 kJ Referensarrow_forwardHow many resonance structures can be drawn for the tellurate ion (TeO42-) in which the central tellurium atom bears a -1 formal charge and the oxygens bear formal charges of either zero or -1? Enter your answer as a whole number. Answer: Enter Value THAarrow_forwardThe arrangement of atoms in several biologically important molecules is given here. Complete the Lewis structures of these molecules by adding multiple bonds and lone pairs. Do not add any more atoms. (a) the amino acid serine: 車 0-H H-C-H H Н—N—с—с—о—н H (b) нон H-N-C-N-H (c) pyruvic acid: ноо н—с—с—с—о—н H. (d) uracil: H (e) carbonic acid: H-0-C-0-Harrow_forward
- II. Please expand each of the following bond-line formulas to the corresponding standard Lewis structure, be sure to show all the bonds and unshared electron pairs as well as formal charges, if it applies. 0: (a) (c) O Br | Br-B-Br -N- (b) HO: NO₂ 0: blos ohsup?arrow_forwardClO2F2+(a) Draw all of the possible Lewis structures (including reasonance structures) of the following compounds.(b) Label the formal charge for each atom.(c) Determine which resonance structure(s) is(are) the better/best and briefly explain. Question 5. Finish the questions regarding the molecule/ion in Question 4 above. (a) Draw the best Lewis structure with a three-dimensional fashion by using dash and solid wedges.(b) Name the electron geometry and molecular geometry of the molecules. Identify the bond angles. Note: For the non-standard bond angels, use “<” or “>” to suggest the deviation of the bond angles from the standard bond angel. (c) Determine wether the molecule is molecule is polar or non-polar. Briefly explain.arrow_forward2- 7. A Lewis electron-dot diagram of the oxalate ion, C₂0₂², is shown. (a) Identify the hybridization of the valence orbitals of either carbon atom in the oxalate ion. (b) Silver oxalate, Ag₂C₂O4(s), is slightly soluble in water. The value of Köp for Ag₂C₂04 is 5,40 × 10-¹². (i) Write the expression for the solubility-product constant, Kp, for Ag₂C₂04. (ii) Calculate the molar solubility of Ag₂C₂O4 in neutral distilled water.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
