
Concept explainers
(a)
Interpretation:Thedashed-wedged line formulas for the isomers of
Concept Introduction:Constitutional isomers are the isomers with same molecular formula but different arrangement of bonded atoms in the molecule. Since they have same molecular formula but different bonding of atoms therefore they exhibit different properties. There are different ways to represent the structural formula such as line formula, wedge formula, condense formula etc.
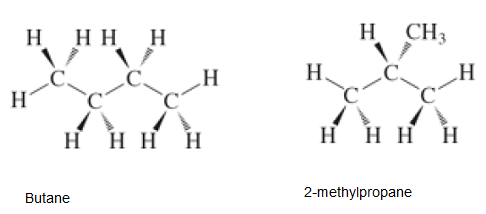
(b)
Interpretation:Thebond line notation for the structure of benzylpenicillin, cubane and saccharine needs to be drawn.
Concept Introduction: Constitutional isomers are the isomers with same molecular formula but different arrangement of bonded atoms in the molecule. Since they have same molecular formula but different bonding of atoms therefore they exhibit different properties. There are different ways to represent the structural formula such as line formula, wedge formula, condense formula etc.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Organic Chemistry: Structure and Function
- (i) Draw the structure of any amine and give the IUPAC name of that amine. (1) Classify the amine in your answer provided in (i) above (iii) Draw the structure of ethyl butanoate and name the functional group. (iv) Give the IUPAC name of the following compound and name the functional group:arrow_forwardZanamivir is an antiviral drug. Identify and circle each functional group (write for example primary amine, secondary alcohol, aldehyde, alkyne, etc.).arrow_forwardCompounds that contain an N-H group associate by hydrogen bonding. (a) Do you expect this association to be stronger or weaker than that of compounds containing an O-H group? (b) Based on your answer to part (a), which would you predict to have the higher boiling point, 1-butanol or 1-butanamine?arrow_forward
- Draw the molecule that corresponds to each IUPAC name. (a) 3,3-dipropoxypentan-1-amine; (b) 2,3,4-trichlorocyclohexanol; (c) 3-cyclopropylpentan-1-ol; (d) 3-(1-methylethyl)cycloheptanaminearrow_forwardDraw each molecule given its name and the following information. (a) Nitroglycerin, also known as 1,2,3-trinitroxypropane, the active ingredient in dynamite and a medication administered to people having a heart attack, (Hint: The nitroxy group is the conjugate base of nitric acid.)arrow_forwardThe structure of Tamiflu, an anti-influenza drug, is shown below (Organic Lett. 2007, 259). Circle and identify each functional group in Tamiflu.arrow_forward
- Draw and name all aromatic compounds with the formula C7H7Clarrow_forward2. a) Draw the structures of Perylene dianhydride, Naphthalene and Salicylic acid b) Arrange the compounds in order of increasing polarity considering the structures and Rf valuesarrow_forwardBiphenyl has the following structure.(a) Is biphenyl a (fused) polynuclear aromatic hydrocarbon?(b) How many pi electrons are there in the two aromatic rings of biphenyl? How does this number compare with that for naphthalene?arrow_forward
- Answer the following :(i) Why is the use of aspartame limited to cold foods and drinks?(ii) How do antiseptics differ from disinfectants?(iii) Why do soaps not work in hard water?arrow_forwardIdentify which of the statements is/are correct. (i) The molecular formula of the smallest aldehyde is C3H6O, and that of the smallest ketone is also C3H6O. (j) The molecular formula of the smallest carboxylic acid is C2H4O2.arrow_forward2. In ordering boiling points, you would likely state, correctly, that, given constant carbon chain length, alkanes have the lowest boiling point, with amines a little higher, and alcohols much higher still. Considering that, for constant R-group, amines and alcohols have pretty much the same molar mass, then why should alcohols, with their O, have higher boiling pints than amines, with their N? Just using class discussions, what is different between them such that their physical properties are so different?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





