
Organic Chemistry: Structure and Function
8th Edition
ISBN: 9781319079451
Author: K. Peter C. Vollhardt, Neil E. Schore
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 1, Problem 55P
Interpretation Introduction
Interpretation:The formal charge on the compound should be chosen.
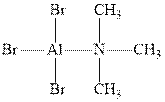
Concept introduction: Formal charge is defined as hypothetical charge present on atom present in molecule. The electrons donated by atom in molecule get positive charge and atom to that electron gets donated get negative charge
Similarly, most organometallic species have polarity that is due to difference in electronegativities of metal an organic species.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the formal charge on the labeled atom (Nitrogen)?
CH,CEN-H
O-2
O+2
O+1
O-1
Which compound has the greatest ionic character?
O RbF
O LiF
O NaF
O CSF
Question 2
Which of the following compound has the central atom with +1 formal
charge?
O PCI6
O XEF3*
O IF5
O AICIA
What is the formal charge on each atom in a hypobromite ion, B r O −?
O = -2, Br = -1
O = -2, Br = +1
O = +1, Br = –2
O = 0, Br = -1
O = -1, Br = 0
Chapter 1 Solutions
Organic Chemistry: Structure and Function
Ch. 1.3 - Prob. 1.1ECh. 1.3 - Prob. 1.2ECh. 1.3 - Prob. 1.3ECh. 1.3 - Prob. 1.4ECh. 1.3 - Prob. 1.5ECh. 1.4 - Prob. 1.6ECh. 1.4 - Prob. 1.8TIYCh. 1.5 - Prob. 1.9ECh. 1.5 - Prob. 1.11TIYCh. 1.6 - Prob. 1.12E
Ch. 1.6 - Prob. 1.13ECh. 1.7 - Prob. 1.15TIYCh. 1.8 - Prob. 1.16ECh. 1.8 - Prob. 1.18TIYCh. 1.9 - Prob. 1.19ECh. 1.9 - Prob. 1.20ECh. 1.9 - Prob. 1.21ECh. 1.9 - Prob. 1.22ECh. 1 - Prob. 25PCh. 1 - Prob. 26PCh. 1 - Prob. 27PCh. 1 - Prob. 28PCh. 1 - Prob. 29PCh. 1 - Prob. 30PCh. 1 - Prob. 31PCh. 1 - Prob. 32PCh. 1 - Prob. 33PCh. 1 - Prob. 34PCh. 1 - Prob. 35PCh. 1 - Prob. 36PCh. 1 - Prob. 37PCh. 1 - Prob. 38PCh. 1 - Prob. 39PCh. 1 - Prob. 40PCh. 1 - Prob. 41PCh. 1 - Prob. 42PCh. 1 - Prob. 43PCh. 1 - Prob. 44PCh. 1 - Prob. 45PCh. 1 - Prob. 46PCh. 1 - Prob. 47PCh. 1 - Prob. 48PCh. 1 - Prob. 49PCh. 1 - Prob. 50PCh. 1 - Prob. 51PCh. 1 - Prob. 52PCh. 1 - Prob. 53PCh. 1 - Prob. 54PCh. 1 - Prob. 55PCh. 1 - Prob. 56PCh. 1 - Prob. 57PCh. 1 - Prob. 58P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Three known isomers exist of N2CO, with the atoms in these sequences: NOCN; ONNC; and ONCN. Write resonance structures for each isomer and use formal charge to predict which isomer is the most stable.arrow_forwardSpecify the formal charges (both sign and magnitude) on the atoms labelled a-c.arrow_forwardWhat is the formal charge on N in this ion? -2 O-1 +1 O +2arrow_forward
- Specify the formal charges (both sign and magnitude) on the atoms labeled a-c.arrow_forwardWhich of the following compound has the central atom with +1 formal charge? O AICI4 O PCI6 O IF5 O XEF3*arrow_forwardDraw a Lewis diagram for BrO4- in which the central Br atom has a formal charge of zero and show all NONZERO formal charges on all atoms. Note that the overall charge on this ion is -1.arrow_forward
- For the following compound, what is the formal charge for the oxygen bonded to the hydrogen. H-O O- O S= N Narrow_forwardIn the cyanide ion (CN-), the nitrogen has a formal charge of O -2 O 1 O -2arrow_forwardFor the carbonate ion (CO32-), there are 3 resonance structures. For any given structure what would the formal charge be for an oxygen that has a single bond to the central carbon atom?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY