
Concept explainers
(a)
Interpretation:The correct Lewis structure by assigning appropriate charge with the help of given bond pair and lone pairs needs to be drawn.
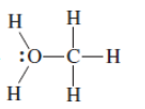
Concept Introduction: Lewis dot structure is also known as Lewis dot formula or electron dot structure. The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons.
Formal charge on each atom can be determined with the help of number of valence shell electrons, number of lone pair electrons and bond pair electrons. The formula for the formal charge can be written as:
(b)
Interpretation: The correct Lewis structure by assigning appropriate charge with the help of given bond pair and lone pairs needs to be drawn.
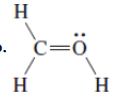
Concept Introduction: Lewis dot structure is also known as Lewis dot formula or electron dot structure. The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons.
Formal charge on each atom can be determined with the help of number of valence shell electrons, number of lone pair electrons and bond pair electrons. The formula for the formal charge can be written as:
(c)
Interpretation: The correct Lewis structure by assigning appropriate charge with the help of given bond pair and lone pairs needs to be drawn.
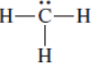
Concept Introduction: Lewis dot structure is also known as Lewis dot formula or electron dot structure. The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons.
Formal charge on each atom can be determined with the help of number of valence shell electrons, number of lone pair electrons and bond pair electrons. The formula for the formal charge can be written as:
(d)
Interpretation: The correct Lewis structure by assigning appropriate charge with the help of given bond pair and lone pairs needs to be drawn.
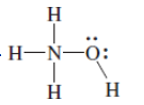
Concept Introduction: Lewis dot structure is also known as Lewis dot formula or electron dot structure. The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons.
Formal charge on each atom can be determined with the help of number of valence shell electrons, number of lone pair electrons and bond pair electrons. The formula for the formal charge can be written as:
(e)
Interpretation: The correct Lewis structure by assigning appropriate charge with the help of given bond pair and lone pairs needs to be drawn.
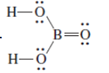
Concept Introduction: Lewis dot structure is also known as Lewis dot formula or electron dot structure. The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons.
Formal charge on each atom can be determined with the help of number of valence shell electrons, number of lone pair electrons and bond pair electrons. The formula for the formal charge can be written as:
(f)
Interpretation: The correct Lewis structure by assigning appropriate charge with the help of given bond pair and lone pairs needs to be drawn.
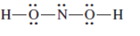
Concept Introduction: Lewis dot structure is also known as Lewis dot formula or electron dot structure. The bond formation between the atoms takes place due to the sharing of valence electrons of bonded atoms while the remaining electrons present in outer shell represented as lone pair of electrons.
Formal charge on each atom can be determined with the help of number of valence shell electrons, number of lone pair electrons and bond pair electrons. The formula for the formal charge can be written as:
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Organic Chemistry: Structure and Function
- A complete Lewis structure must show all nonzero formal charges. Complete each of thefollowing Lewis structures by adding any missing formal charges.arrow_forwardComplete the following Lewis structure by adding in missing lone pairs and pi bonds. Assume all atoms are neutral (having no formal charge). Do not add additional atoms to the structure.arrow_forwardWhen drawing a resonance hybrid, how is the relative significance of individual resonance structures represented? O The entire resonance structure will be drawn larger or smaller, depending on its significance. O There is no way to note the significance of a resonance structure in atdrawing. The most significant resonance structure is circled. The partial charges will be written larger or smaller, depending on the significance of the resonance structure. Save for Later Submisarrow_forward
- Please refer to the example image to answer. You must use CER, claim-evidence-reasoning. Make sure that your answer is CLEAR and explained well. Claim is your answer to the question. Evidence is from the image and reasoning is your explanation. Proper evidence for all Lewis structures include: Most electronegative atom must be in the center. Octet of electrons surrounding each atom. Total number of electrons depicted equals same total number of valence electrons from each participating atom. Make sure to refer to the example image because it shows the correct Lewis structures. The question you're answering is about the students drawing of CH20.arrow_forwardFor each of the following molecules draw the Lewis structure on a separate sheet of paper. MAKE SURE TO FOLLOW THE RULES FROM CLASS (ie do not break the octet rule unless necessary to connect all the atoms). These can all be drawn on one piece of paper. Based on your structure provide the following: the total number of valence electrons. a picture of the Lewis structure (3-D model not required). the total number of structural domains around the CENTRAL atom. the number of bonding domains around the CENTRAL atom. the number of lone pairs around the CENTRAL atom. the electronic and molecular shapes. the hybridization of the CENTRAL atom. (sp, sp2, sp3, sp3d, or sp3d2) whether or not the molecule is polar (Enter Y for yes and N for no). Note: The central atom is the first atom listed.arrow_forwardParaphrasing .ewriting Tool Car note Pirate Ship BLACKBOARD [Review Toplcs) [References) Unshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. The number of unshared pairs at atom a is 0 0 b c CH,-CH2-0-H a The number of unshared pairs at atom b is 3 0. The number of unshared pairs at atom c is o e. The number of unshared pairs at atom a is The number of unshared pairs at atom b is „CH2 The number of unshared pairs at atom c is Submit Answer Retry Entire Group 9 more group attempts remaining (Previous Next> FE 1 18 étv S ali Z W MacBook Air DII 888 F10 FO F3 F4 & dele 4 6. 8 { P R Y F G J K L V M command option Barrow_forward
- Write a Lewis structure for each of the following molecules that are exceptions to the octet rule. ClO2 Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons and nonbonding electrons. Do not include charges.arrow_forward< Complete the following structural formula for a neutral molecule by adding H atoms to complete the valence of each atom. Do not introduce any double or triple bonds. Then complete the Lewis diagram by adding any unshared electron pairs needed, so that each atom except H has a complete octet. [Review Topics] [References] Use the References to access important values if needed for this question. Br Br C—C— Write the molecular formula in the order CHX, where X stands for Cl or Br. Submit Answer The number of unshared pairs in the Lewis diagram unshared pair(s). Retry Entire Group 9 more group attempts remaining Previous Email Instructor Next Save and Earrow_forwardExplaine why, in Part I, the formal charge is equal to the number of valence electrons. Match the words in the left column to the appropriate blanks in the sentences on the right. minus The formal charge on each atom is the number of valence electrons the number of plus lone pair electrons and the number of bonding electrons. The formal charge is equal to the number of valence electrons because there are bonds. one-half two times no multiplearrow_forward
- How do you know when to draw a solid wedge vs a dashed wedge when drawing 3D bond-line structures? I know that solid-wedge means the atom is pointing towards you and dashed wedge means it's in the back, but how do you know which atoms are in the front as opposed to the back? How can you tell what the configuration will look like in space just by looking at the lewis structure or name?arrow_forwardDraw three resonance structures for CS,. This species has its three atoms bonded sequentially in the following fashion: S-C-S. Draw your resonance structures so that the atoms in them are bonded together in this order. Select the most important resonance structure for this species based on the formal charges on the atoms of the three resonance structures you have drawn. Select the choices from below which make the statements true about this (most important) resonance structure. (a) The leftmost bond (between S and C) is a single v bond. (b) The rightmost bond (between C and S) is a single v bond. (c) The formal charge on the leftmost (S) atom is -Select-v (d) The formal charge on the central (C) atom is -Select---v (e) The formal charge on the rightmost (S) atom is Select-v (f) The number of nonbonding pairs (lone pairs) of electrons in the leftmost (S) atom is Select-v pairs. (g) The number of nonbonding (lone) pairs of electrons in the rightmost (S) atom is -Select-- v pairs.arrow_forwardDraw the resonance structure indicated by the curved arrows. Assign formal charges. H H/:O: H H-C-C-C-Ċ—H H H H Draw the molecule by placing atoms on the canvas and connecting them with bonds. Include all hydrogen atoms and nonbonding electrons. Show the formal charges of all atoms in the correct structure.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

