
Concept explainers
(a)
Interpretation:Condensed formula that shows multiple bonds, lone pairs should be drawn for the pair
Concept introduction: There are three ways of representation of molecular structures. The hashed and wedged formulas have three types of bonds. The bond above the plane is represented by bold hash while bonds behind the plane are represented by wedge. The bonds in plane are represented by normal bonds.
Bond-line formula depicts the zig-zag framework of carbon chain with attached substituents. The terminal position represents a methyl while the non-terminal is for methylene group. For instance, the propane is written in this notation as follows:
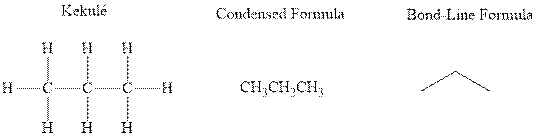
(b)
Interpretation: Condensed formula that shows multiple bonds, lone pairs should be drawn for the pair
Concept introduction: There are three ways of representation of molecular structures. The hashed and wedged formulas have three types of bonds. The bond above the plane is represented by bold hash while bonds behind the plane are represented by wedge. The bonds in plane are represented by normal bonds.
Bond-line formula depicts the zig-zag framework of carbon chain with attached substituents. The terminal position represents a methyl while the non-terminal is for methylene group. For instance the propane is written in this notation as follows:
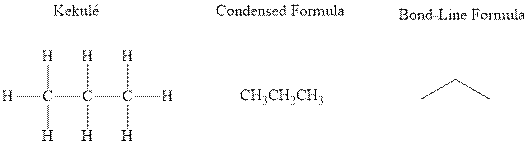
(c)
Interpretation: Condensed formula that shows multiple bonds, lone pairs should be drawn for the pair
Concept introduction: There are three ways of representation of molecular structures. The hashed and wedged formulas have three types of bonds. The bond above the plane is represented by bold hash while bonds behind the plane are represented by wedge. The bonds in plane are represented by normal bonds.
Bond-line formula depicts the zig-zag framework of carbon chain with attached substituents. The terminal position represents a methyl while the non-terminal is for methylene group. For instance the propane is written in this notation as follows:
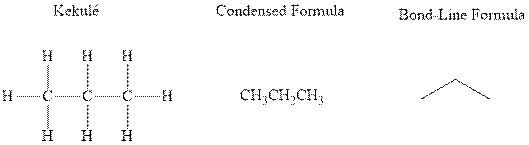
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Organic Chemistry: Structure and Function
- Structural Isomers: In the next part you will learn a little about structural isomers and how to draw them. Structural isomers are compounds with the same molecular formula, but different bonding arrangements or connectivities. In other words, the atoms are connected in a different order. For example, there are 2 different compounds with the molecular formula C4H10. They are given below: H H-C- H -H H H HHH H- H H-C -H Η ΗΗ H H Do you see how the atoms are bonded in a different order in the 2 structures? So they are structural isomers. C3H12 Find three different molecular structures, isomers, with this formula. (Hint: Remember the C rule where every C atom needs to have 4 bonds.) C2H60 Find two different molecules with this formula. (Hint: What are the 2 ways you can C5H10 How many structural isomers can you find for this formula? (Hint: Notice that 2 H atoms have been removed. This means that there is EITHER 1 double bond OR there is a ring structure.) Try to provide at least 4…arrow_forwardThere are at least three different molecules with the formula C3H8O. Draw a Lewis structure for each possible constitutional isomer. Be careful not to duplicate any structures.arrow_forwardAnswer the questions in the table below about the shape of the methanone (H₂CO) molecule. How many electron groups are around the central carbon atom? Note: one "electron group" means one lone pair, one single bond, one double bond, or one triple bond. What phrase best describes the arrangement of these electron groups around the central carbon atom? (You may need to use the scrollbar to see all the choices.) (choose one) X Sarrow_forward
- Two major resonance structures are possible for the anion shown. One resonance form is given, but it is incomplete. Complete the given structure by adding nonbonding electrons and formal charges. Draw the remaining structure, including nonbonding electrons and formal charges. Omit curved arrows. Structure A: complete the structure by adding nonbonding electrons and formal charges. H H H I Structure B: draw the remaining resonance structure, including nonbonding electrons and formal charges. H- : z: H Harrow_forwardWhich molecule contains carbon with a negative formal charge? CO CO2 H2CO CH4arrow_forwardAfter drawing the Lewis dot structure for CH3CI determine the number of single bond(s), double bond(s), and lone pair(s) on the central atom. single bond(s) = 4, double bond(s) = 0, lone pair(s) = 0 single bond(s) = 4, double bond(s) = 0, lone pair(s) = 1 single bond(s) = 4, double bond(s) = 1, lone pair(s) = 0 single bond(s) = 3, double bond(s) = 0, lone pair(s) = 1 single bond(s) = 3, double bond(s) = 1, lone pair(s) = 0arrow_forward
- C2H4 a) How many lone pairs (non-bounding electron pairs) does the compound possess on All atoms? (central atom(s) and outer atoms? b) For this compound, Identify the following -number of electron groups (electron domains) -number of atoms bounded to the central atom -number of non-bounding electron pairs (lone pairs) attached to the central atom -General formula c) What is the Electron group Geometry also called "overall shape" and Hybrid Orbital designation for the central atm? d)What is the shape the atoms make (excluding the lone pairs)? This is also called thearrow_forwardC2H2 a) How many lone pairs (non-bounding electron pairs) does the compound possess on All atoms? (central atom(s) and outer atoms? b) For this compound, Identify the following -number of electron groups (electron domains) -number of atoms bounded to the central atom -number of non-bounding electron pairs (lone pairs) attached to the central atom -General formula c) What is the Electron group Geometry also called "overall shape" and Hybrid Orbital designation for the central atm? d)What is the shape the atoms make (excluding the lone pairs)? This is also called the "molecular shape"arrow_forwardTwo or more of your answers are incorrect. Use this condensed chemical structure to complete the table below. O || CH,=CH–C-H The condensed chemical structure of acrolein Some facts about the acrolein molecule: number of carbon-carbon single (CC) bonds: number of carbon-hydrogen single (CH) bonds: number of lone pairs: X S 2 1 4arrow_forward
- Directions CHCЬ CH3CI CCla Write number of valence electrons for cach atom and find the total number of valence electrons Determine center atom and draw the skeleton Write the full Lewis Structure with all valence electrons Determine non-zero formal charges Center atom has regions Name the electronic geometry Center atom: # bonding atoms? # lone pairs? Name the molecular gcometry Draw the molecular geometry Identify polar covalent pairs. & Add dipole arrows for each polar bond Is there a net dipole? Is the molecule Polar or Nonpolar?arrow_forwardIn the following Lewis structure of [(CH3)2OH]*, every atom, bond and lone pair is positioned. To complete the structure, drag the formal charge tags to the appropriate atom(s). Each marker may be used more than once, or not at all. If an atom has a formal charge of zero, do not drag a tag to it. When you drag the marker in, place the little crosshairs in the upper left corner of the marker directly over the atom(s) in question (not above them). H. H-C Н-С-О-С-Н C-H H HH 2- II 2-arrow_forwardH-N=C-N-H 1 Draw the major contributing structure with formal charge(s) below. If there are electrons around any of the atoms ignore them for now. The details will be addressed in the next section. Draw Your Solution Now specify the number of electrons in lone pairs around the specified atoms in the contributing structure that was drawn. The atom labels correspond to the atom labels on the structure in the question. N1 has 2 N2 has C1 and C C3 and C C5 and C H In the contributing structure, the double bonds are placed between: C1 has C2 has C3 has C4 has C5 has C6 has electron(s). electron(s). Now specify the number of electrons in lone pairs around the specified atoms in the contributing structure. The atom labels correspond to the atom labels on the structure in the question. New WP Integrat...pdf electron(s). electron(s) electron(s). electron(s). electron(s). electron(s)arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


