
Concept explainers
(a)
Interpretation:Constitutional isomer possible for
Concept introduction:There are three ways of representation of molecular structures. The hashed and wedged formulas have three types of bonds. The bond above the plane is represented by bold hash while bonds behind the plane are represented by wedge. The bonds in plane are represented by normal bonds.
Bond-line formula depicts the zig-zag framework of carbon chain with attached substituents. The terminal position represents a methyl while the non-terminal is for methylene group. For instance, the propane is written in this notation as follows:
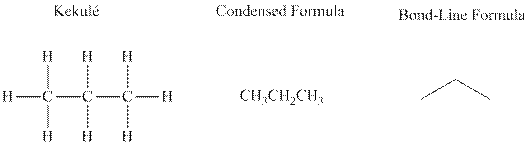
Molecules that possess identical molecular formula but vary in the manner of their connectivity of the bonds are referred to as structural isomers. These are essentially found in
For instance, the smallest branched alkane possible is

The two compounds, in fact, constitute a pair of constitutional isomers. These kinds of isomers are formed by cleavage and replacement of bonds, unlike the conformational isomers that result due to rotation around the bond.The structural isomers possible for a given alkane increase rapidly with a number of carbon atoms. This is justified as for alkanes higher than propane more ways exist to connect the carbon atoms.
(b)
Interpretation: Constitutional isomer possible for
Concept introduction:There are three ways of representation of molecular structures. The hashed and wedged formulas have three types of bonds. The bond above the plane is represented by bold hash while bonds behind the plane are represented by wedge. The bonds in plane are represented by normal bonds.
Bond-line formula depicts the zig-zag framework of carbon chain with attached substituents. The terminal position represents a methyl while the non-terminal is for methylene group. For instance, the propane is written in this notation as follows:
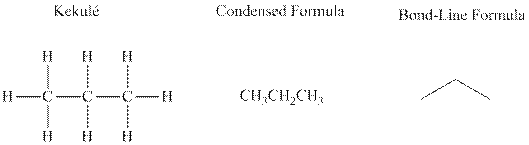
Molecules that possess identical molecular formula but vary in the manner of their connectivity of the bonds are referred to as structural isomers. These are essentially found in alkanes that can be branched by removal of
For instance, the smallest branched alkane possible is

The two compounds, in fact, constitute a pair of constitutional isomers. These kinds of isomers are formed by cleavage and replacement of bonds, unlike the conformational isomers that result due to rotation around the bond.The structural isomers possible for a given alkane increase rapidly with a number of carbon atoms. This is justified as for alkanes higher than propane more ways exist to connect the carbon atoms.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Organic Chemistry: Structure and Function
- Draw the shapes of the following molecules and ions in 3-dimension. Show clearly any lone pairs of electrons on the central atom, state the number of bond pairs and lone pairs of electrons on the central atom and name the shape of the molecule or ion. (a) CH3+, methyl carbocation (b) HOBr, bromic (I) acid (c) NCl3, nitrogen trichloride Provide everything stated in the instructions for each compound.arrow_forwardIn the following compounds, the C atoms form a single ring.Draw a Lewis structure for each compound, identify cases for which resonance exists, and determine the carbon-carbon bondorder(s): (a) C₃H₄; (b) C₃H₆; (c) C₄H₆; (d) C₄H₄; (e) C₆H₆.arrow_forward(a) Determine the formal charge of oxygen in the following structure. If the atom is formally neutral, indicate a charge of zero. (b) Draw an alternative Lewis (resonance) structure for the compound given in part (a). Show the unshared pairs and nonzero formal charges in your structure. Don't use radicals. Formal charge on O 0arrow_forward
- Help me pleasearrow_forwardDetermine a molecular formula, e.g. CH4, from the line structure below. Specify elements in the following order: C, H, others(in alphabetical order). Example: C4H7ClOS Molecular formulaarrow_forwardOne way of naming alcohols is to name the alkyl group that is attached to the −OH and add the word alcohol. Write bond-line formulas for (a.) propyl alcohol and (b.) isopropyl alcohol.arrow_forward
- Convert each of the following molecular models into a skeletal structure, and give the formula of each. Only the connections between atoms are shown; multiple bonds are not indicated (gray = C, red = O, blue = N, ivory = H).arrow_forwardDraw all of the resonance structures for each of the following species. Be sure to include the curved arrows that indicate which pairs of electrons are shifted in going from one resonance structure to the next. Draw the resonance hybrid of each species. (a) ОН (b) (c) H3Carrow_forwardDraw the shapes of the following molecules and ions in 3-dimension. Show clearly any lone pairs of electrons on the central atom, state the number of bond pairs and lone pairs of electrons on the central atom and name the shape of the molecule or ion. (a) SiCI4, silicon tetrachloride (b) PBr3, phosphorus tribromide (c) CI2O, dichlorine oxide Provide everything stated in the instructions for each compound.arrow_forward
- 13) Give the correct bond-line structure for the following compounds (drawing bonds as lines). Clearly show all bonds, electron pairs, and formal charges when present. Ionic bonds, if present, must be clearly marked using formal charges. (CH3)2CHCHBrOH Na o N oarrow_forwardQ. 9a) Provide additional resonance structures for the following species. Remember to show lone pairs & formal charges where applicable + [ :N=N-F: +→ [:0-C!-O: → Q. 9b) Draw the Lewis Structure for NCCH2CO2NHCH3, determine the number of sigma bonds & the number of pi bonds present, and indicate the bond angle value around each carbon atom, each nitrogen atom, and each oxygen atom.arrow_forwardHow do a C= O and a C= C compare with regards to the following: (a) geometry; (b) polarity; (c) type of reaction?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





