
Concept explainers
(a)
Interpretation:TheKekulè (straight line) notation for the given condensed formula needs to be determined.
Concept Introduction:The chemical compounds can be shown in different structural formulae. Condense formula, wedge-dashed formula and bond-line formula are some common ways to show the structural of chemical compounds.
In wedge-dashed line formula, the groups or atoms bonded to each C atoms must be shown with dashed and wedges. Here wedge is the solid line that represents the bond in plane of the surface whereas dash line represents the line extended back behind the surface.
In the bond line formula, each C is shown as dot and the each dot is connected with another dot with line. Excluding C and H, all other elements of the compound like N, S, O must be indicted with their atomic symbols. In Kekulè (straight line) notation all atoms and
(b)
Interpretation: The Kekulè (straight line) notation for the given condensed formula needs to be determined.
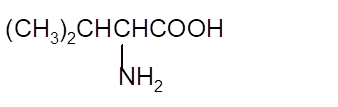
Concept Introduction:The chemical compounds can be shown in different structural formulae. Condense formula, wedge-dashed formula and bond-line formula are some common ways to show the structural of chemical compounds.
In wedge-dashed line formula, the groups or atoms bonded to each C atoms must be shown with dashed and wedges. Here wedge is the solid line that represents the bond in plane of the surface whereas dash line represents the line extended back behind the surface.
In the bond line formula, each C is shown as dot and the each dot is connected with another dot with line. Excluding C and H, all other elements of the compound like N, S, O must be indicted with their atomic symbols. In Kekulè (straight line) notation all atoms and chemical bonds must be shown with any symbol of lone pairs on atoms if any.
(c)
Interpretation: The Kekulè (straight line) notation for the given condensed formula needs to be determined.
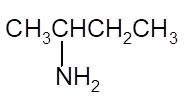
Concept Introduction:The chemical compounds can be shown in different structural formulae. Condense formula, wedge-dashed formula and bond-line formula are some common ways to show the structural of chemical compounds.
In wedge-dashed line formula, the groups or atoms bonded to each C atoms must be shown with dashed and wedges. Here wedge is the solid line that represents the bond in plane of the surface whereas dash line represents the line extended back behind the surface.
In the bond line formula, each C is shown as dot and the each dot is connected with another dot with line. Excluding C and H, all other elements of the compound like N, S, O must be indicted with their atomic symbols. In Kekulè (straight line) notation all atoms and chemical bonds must be shown with any symbol of lone pairs on atoms if any.
(d)
Interpretation: The Kekulè (straight line) notation for the given condensed formula needs to be determined.
Concept Introduction:The chemical compounds can be shown in different structural formulae. Condense formula, wedge-dashed formula and bond-line formula are some common ways to show the structural of chemical compounds.
In wedge-dashed line formula, the groups or atoms bonded to each C atoms must be shown with dashed and wedges. Here wedge is the solid line that represents the bond in plane of the surface whereas dash line represents the line extended back behind the surface.
In the bond line formula, each C is shown as dot and the each dot is connected with another dot with line. Excluding C and H, all other elements of the compound like N, S, O must be indicted with their atomic symbols. In Kekulè (straight line) notation all atoms and chemical bonds must be shown with any symbol of lone pairs on atoms if any.
(e)
Interpretation: The Kekulè (straight line) notation for the given condensed formula needs to be determined.
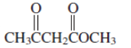
Concept Introduction:The chemical compounds can be shown in different structural formulae. Condense formula, wedge-dashed formula and bond-line formula are some common ways to show the structural of chemical compounds.
In wedge-dashed line formula, the groups or atoms bonded to each C atoms must be shown with dashed and wedges. Here wedge is the solid line that represents the bond in plane of the surface whereas dash line represents the line extended back behind the surface.
In the bond line formula, each C is shown as dot and the each dot is connected with another dot with line. Excluding C and H, all other elements of the compound like N, S, O must be indicted with their atomic symbols. In Kekulè (straight line) notation all atoms and chemical bonds must be shown with any symbol of lone pairs on atoms if any.
(f)
Interpretation: The Kekulè (straight line) notation for the given condensed formula needs to be determined.
Concept Introduction:The chemical compounds can be shown in different structural formulae. Condense formula, wedge-dashed formula and bond-line formula are some common ways to show the structural of chemical compounds.
In wedge-dashed line formula, the groups or atoms bonded to each C atoms must be shown with dashed and wedges. Here wedge is the solid line that represents the bond in plane of the surface whereas dash line represents the line extended back behind the surface.
In the bond line formula, each C is shown as dot and the each dot is connected with another dot with line. Excluding C and H, all other elements of the compound like N, S, O must be indicted with their atomic symbols. In Kekulè (straight line) notation all atoms and chemical bonds must be shown with any symbol of lone pairs on atoms if any.
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Organic Chemistry: Structure and Function
- Classify each of these chemical compounds: compound HI CH, CH₂OH PbCl₂2 type of compound (check all that apply) molecular ionic organic inorganic hydrocarbon molecular ionic organic inorganic hydrocarbon molecular ionic organic inorganic hydrocarbonarrow_forwardSelect all functional groups that are present in the molecule shown. H3CO. carboxylic acid ☐aldehyde amide alkyl halide ether ☐ alcohol ☐ ketone ☐alkene ☐aldehyde ☐amine O ester Br O Br OCH 3arrow_forwardThe name of the compound below is H3C CH2 CH3arrow_forward
- Classify each of these chemical compounds: compound CH, (CH₂)₂OH ZnI₂ CH₂CH₂CH3 I Don't Know type of compound (check all that apply) molecular Submit 00 00000 0.0 X ionic organic inorganic hydrocarbon molecular ionic organic inorganic hydrocarbon molecular ionic organic inorganic hydrocarbonarrow_forward2. CH,CH;CH,CH,Br CH,CH,CCH,arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





