
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Please refer to the example image to answer. You must use CER, claim-evidence-reasoning. Make sure that your answer is CLEAR and explained well.
Claim is your answer to the question. Evidence is from the image and reasoning is your explanation.
Proper evidence for all Lewis structures include:
Most electronegative atom must be in the center.
Octet of electrons surrounding each atom.
Total number of electrons depicted equals same total number of valence electrons from each participating atom.
Make sure to refer to the example image because it shows the correct Lewis structures. The question you're answering is about the students drawing of CH20.

Transcribed Image Text:A student was asked to draw the Lewis structure for CH20. Is the Lewis structure the student drew below
correct?
:H:
:0:
I
H:
Why or why not? Be sure to support your claim with evidence and reasoning. Write your response in complete
sentences.
Transcribed Image Text:H
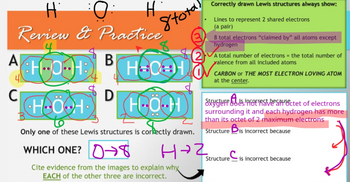
Review & Practice
B
HOOOH
HOH
A
C
Hi
D
Stotal
H-CH
HÖ•H
Only one of these Lewis structures is correctly drawn.
WHICH ONE? →
H→2
Cite evidence from the images to explain why
EACH of the other three are incorrect.
Correctly drawn Lewis structures always show:
Lines to represent 2 shared electrons
(a pair)
8 total electrons "claimed by" all atoms except
hydrogen
2A total number of electrons = the total number of
yalence from all included atoms
CARBON or THE MOST ELECTRON LOVING ATOM
at the center.
skygturistesa ctet of electrons
surrounding it and each hydrogen has more
than its octet of 2 maximum electrons
Structure Bis incorrect because
Structure is incorrect because
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Analysis HF - The ball and stick structure for HF is shown. Answer the following and do what is asked Which atom is more electronegative? Draw a bond polarity arrow (bond dipole) Draw the partial charges on the molecule Would you expect this to move in an electric field? Draw it in the field provided. H2O - The ball and stick structure for H2O is given. Answer the following and do what is asked. Which atom is more electronegative? H F Draw a bond polarity arrow (bond dipole) Place partial charges on the molecule In a different color draw a molecular dipole arrow. Would you expect this to move in an electric field? Draw it in the field provided. SCH4U: 1-5 VSEPR Shape and Polarity Lab ROSEDALE 0 ROSEDALE GLOBAL HIGH SCHOOL Name: CO2 - The ball and stick structure for CO 2 is given. Answer the following and do what is asked. Which atom is more electronegative? Draw a bond polarity arrow (bond dipole) Place partial charges on the molecule In a different color draw a molecular dipole…arrow_forwardPlease do it correctly (posting second time) pleasearrow_forwardQuestion 1 The Lewis structure of an ion is given above. Answer the following questions for this ion. a) Determine the formal charge on the (P) atom. b) Write the name of the molecular shape. c) What is the total number of electrons in the Lewis structure? d) How many electron groups does the central atom have? e) Write the VSEPR notation and indicate the meaning of each symbol in this notation. f) Write the name of the electron-group geometry. g) What type of hybrid orbitals does the central atom have?arrow_forward
- Chemistry Review | Constants | Periodic Table KCN Draw the molecule on the canvas by choosing buttons from the Tools (for bonds and charges), Atoms, and Templates toolbars. H CEN * Incorrect; Try Again At least one of the atoms in your response has an invalid valence. If you don't see an atom highlighted in red, look for an atom that violates the octet rule, or try expanding your shortcut groupsarrow_forwardUse the Table of Electronegativities provided to answer the following question. What kind of bond exists between O and H? primarily ionic nonpolar covalent polar covalentarrow_forwardWhy are atoms in an ionic compound attracted to each other . Multiple choice in image .arrow_forward
- Draw the Lewis structure for OSiS before answering the following questions (You do NOT submit a picture just answer the questions). Silicon is the central atom and all atoms obey the octet rule. WARNING: Do NOT USE the internet or other sources to find the structures. Use ONLY the rules taught in class. ALL atoms obey the octet rule Answer the following questions for the Lewis structure for OSiS , given that silicon is the central atom and all atoms obey the octet rule. Give answers as numbers (1,2,3 ...etc.) NOT words (one, two three etc.) How many double bonds exist in this structure How many electrons surround the silicon atom How many lone pairs are around the silicon atom How many lone pairs are around the sulfur atom How many lone pairs are around the oxygen atom How many electrons surround the Sulfur atomarrow_forwardDraw the lewis dot diagram for iodine monochloride. How many total lone pairs of electrons are in this molecule?arrow_forwardHydrogen is in Group 1 of the periodic table. Which kind of bond would form between two hydrogens? O An ionic bond would form because both atoms are nonmetals. A covalent bond would form because the electron would be shared so both hydrogens have a fullI, stable shell. An ionic bond would form because one hydrogen would transfer its valence electron to the other hydroger make a full shell. O A metallic bond would form because both atoms are metals. P Type here to search の 近arrow_forward
- Counting available electrons and drawing a Lewis structuresarrow_forwardUse Lewis theory to determine the formula for the compound that forms between each of the following pairs of elements. Ca and Te Express your answer as a chemical formula. Mg and Br Express your answer as a chemical formula. Na and S Express your answer as a chemical formula. In and O Express your answer as a chemical formula.arrow_forwardPart A For each formula, fill in the eight items listed in the instructions. The Lewis models (at least) should be completed before coming to lab. Be sure to consider the Lewis dot model and the ball-and-stick model as two separate items. The Lewis model should not attempt to indicate geometry. The ball-and-stick model does not need to show multiple bonds or the positions of lone pairs of electrons but should depict molecular geometry. Since ions have a net charge, the issue of polarity is usually unimportant for them. You can skip the polarity analysis for the ions. Fill in the all blanks!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY