
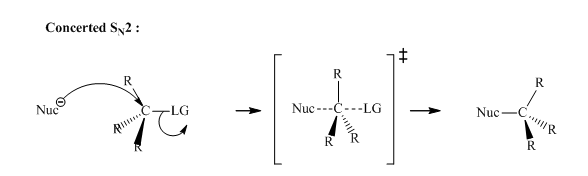
Concept explainers
Interpretation:The significance of kinetic data for reactions of
Concept introduction: Bimolecular substitution or
A general
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic Chemistry: Structure and Function
- CH3 CH3 Br- Br2 CH2CI2 CH3 CH3 H3C H3C Br Electrophilic addition of bromine, Br2, to alkenes yields a 1,2-dibromoalkane. The reaction proceeds through a cyclic intermediate known as a bromonium ion. The reaction occurs in an anhydrous solvent such as CH2C12. In the second step of the reaction, bromide is the nucleophile and attacks at one of the carbons of the bromonium ion to yield the product. Due to steric clashes, the bromide ion always attacks the carbon from the opposite face of the bromonium ion so that a product with anti stereochemistry is formed. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions CH3 CH3 Br- .CH3 .CH3 H3C H3C :Br :Br:arrow_forward5-The hydrolysis of a series of ethyl benzoates by hydroxide ion in 85% aqueous ethanol has been investigated. A Hammett plot of the second order rate constants (K) gave a reaction constant p = 2.56. Calculate how much faster ethyl 4- nitrobenzoate will undergo base catalyzed hydrolysis compared to ethyl benzoate under similar conditions. O,Narrow_forwardConsider this reaction: Br CH;OH Br-Br H3CO The mechanism proceeds through a first cationic intermediate, intermediate 1. Nucleophilic attack leads to intermediate 2, which goes on to form the final product. In cases that involve a negatively charged nucleophile, the attack of the nucleophile leads directly to the product. Br + CH3OH Br Intermediate 1 Intermediate 2 (product) In a similar fashion, draw intermediate 1 and intermediate 2 (final product) for the following reaction. OH + Br2 + HBr Br racemic mixturearrow_forward
- Consider this reaction: Br CH3OH Br-Br H3CO The mechanism proceeds through a first cationic intermediate, intermediate 1. Nucleophilic attack leads to intermediate 2, which goes on to form the final product. In cases that involve a negatively charged nucleophile, the attack of the nucleophile leads directly to the product. H. Br + CH;OH Br Intermediate 2 (product) Intermediate 1 In a similar fashion, draw intermediate 1 and intermediate 2 (final product) for the following reaction. OH + Br2 + HBr Br racemic mixturearrow_forwardTRUE or FALSE: For a transition state to be considered as the rate- determining step (rds) in a multi-step reaction, it must have the highest change in enthalpy.arrow_forward6. Which of the following expressions is representative of the rate law for a Sy2 reaction ? 'N² k [electrophile] (b) Rate =k [electrophile] [nucleophile] (d) Rate = k[electrophile]? (a) Rate = (c) Rate =k [nucleophile]2arrow_forward
- Consider the given reaction in which NC−NC− is the nucleophile and CH3CNCH3CN is the solvent. The reactant molecule has a structure with solid and dashed wedge bonds. A solid wedge () is used to show the bond that is above the plane of the paper, and a dashed wedge () is used to show the bond that is behind the plane of the paper. Draw the product of the following reaction:arrow_forwardThe reaction of ozone with 2-butene leads to -10 formation of aldehyde + O ketones, aldehyde + alcohol, two molecules of carboxylic acid O.arrow_forwardIn unpolluted air at 300 K, the hydroxyl radical OH reacts with CO with a bimolecular rate constant of 1.6 × 101' L mol-1 s-1 and with CH4 with a rate constant of 3.8 × 10° L mol¬1 s-1. Take the partial pressure of CO in air to be constant at 1.0 × 10-7 atm and that of CH4 to be 1.7 x 10-6 atm, and assume that these are the primary mecha- nisms by which OH is consumed in the atmosphere. Cal- culate the half-life of OH under these conditions.arrow_forward
- The following is a nucleophilic substitution reaction of S-3-chloro-2-methylhexane with "CN. The experimental rate law for this reaction is Rate = k [S-3-chloro-2-methylhexane] CH3 H,C. CH3 + CEN H2 The mechanism for this reaction is SN1 HCarrow_forwardPleasearrow_forwardPericyclic Reactions: Convert 1a to 1b Indicate what type of reactions and mechanisms occured in each step. Indicate reagents/conditions.arrow_forward
