
(a)
Interpretation:The rate constant for reaction between
Concept introduction: Bimolecular substitution or
Bimolecular substitution reaction depend concentration of both the
A general
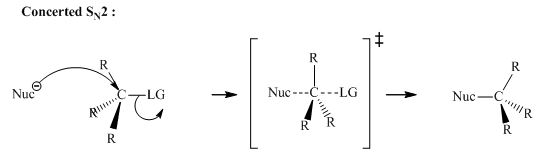
Polar-aprotic solvents accelerate the rate of
(a)
Interpretation:The initial rates for reaction between
Concept introduction: Bimolecular substitution or
A general
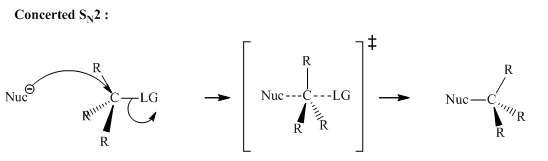
Bimolecular substitution reaction depend concentration of both the alkyl halide and nucleophile. Hence the name
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic Chemistry: Structure and Function
- The label on a bottle of 3% (by volume) hydrogen peroxide, H2O2, purchased at a grocery store, states that the solution should be stored in a cool, dark place. H2O2decomposes slowly over time, and the rate of decomposition increases with an increase in temperature and in the presence of light. However, the rate of decomposition increases dramatically if a small amount of powdered MnO- is added to the solution. The decomposition products are H2O and O2. MnO2 is not consumed in the reaction. Write the equation for the decomposition of H2O2. What role does MnO2 play? In the chemistry lab, a student substituted a chunk of MnO2 for the powdered compound. The reaction rate was not appreciably increased. WTiat is one possible explanation for this observation? Is MnO2 part of the stoichiometry of the decomposition of H2O2?arrow_forward7-35 A reaction has a high rate constant but a small equilibrium constant. What does this mean in terms of producing an industrial product?arrow_forwardThe Raschig reaction produces the industrially important reducing agent hydrazine, N2H4, from ammonia, NH3, and hypochlorite ion, OCl−, in basic aqueous solution. A proposed mechanism is Step 1: Step 2: Step 3: What is the overall stoichiometric equation? Which step is rate-limiting? What reaction intermediates are involved? What rate law is predicted by this mechanism?arrow_forward
- . Account for the increase in reaction rate brought about by a catalyst.arrow_forwardThe rate constant, k, at 25 C is 0.27/h for the reaction Pt(NH3)2Cl2(aq) + H2O() [Pt(NH3)2(H2O)Cl]+(aq) + Cl(aq) and the rate equation is Reaction rate = k[Pt(NH3)2C12] Calculate the rate of reaction when the concentration of Pt(NH3)2Cl2 is 0.020 M.arrow_forward(Section 11-5) A rule of thumb is that for a typical reaction, if concentrations are unchanged, a 10-K rise in temperature increases the reaction rate by two to four times. Use an average increase of three times to answer the questions below. (a) What is the approximate activation energy of a typical chemical reaction at 298 K? (b) If a catalyst increases a chemical reactions rate by providing a mechanism that has a lower activation energy, then what change do you expect a 10-K increase in temperature to make in the rate of a reaction whose uncatalyzed activation energy of 75 kJ/mol has been lowered to one half this value (at 298 K) by addition of a catalyst?arrow_forward
- . explain the difference between elementary reactions and multistep reactions.arrow_forwardDiethylhydrazine reacts with iodine according to the following equation: Â (C2H5)2(NH)2(l)+I2(aq)(C2H5)2N2+2HI(aq)The rate of the reaction is followed by monitoring the disappearance of the purple color due to iodine. The following data are obtained at a certain temperature. (a) What is the order of the reaction with respect to diethylhydrazine, iodine, and overall? (b) Write the rate expression of the reaction. (c) Calculate k for the reaction. (d) What must [(C2H5)2] be so that the rate of the reaction is 5.00104mol/Lh when [ I2 ]=0.500M?arrow_forwardDefine stability from both a kinetic and thermodynamic perspective. Give examples to show the differences in these concepts.arrow_forward
- One possible mechanism for the decomposition of nitryl chloride, NO2CI, is What is the overall reaction? What rate law would be derived from this mechanism? What effect does increasing the concentration of the product NO2 have on the reaction rate?arrow_forwardGive at least two physical properties that might be used to determine the rate of a reaction.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





