
(a)
Interpretation: The mechanism by curved-arrow notation for below reaction should be written.
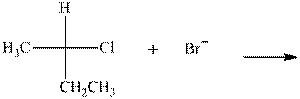
Concept introduction:Bimolecular substitution or
A general
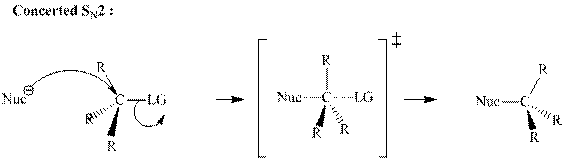
(b)
Interpretation:The mechanism by curved-arrow notation for below reaction should be written.
Concept introduction:Bimolecular substitution or
A general
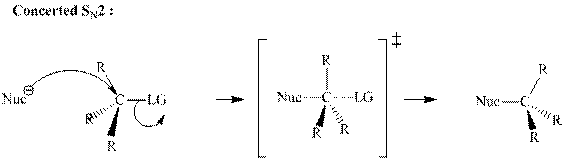
(c)
Interpretation:The mechanism by curved-arrow notation for below reaction should be written.
Concept introduction:Bimolecular substitution or
A general
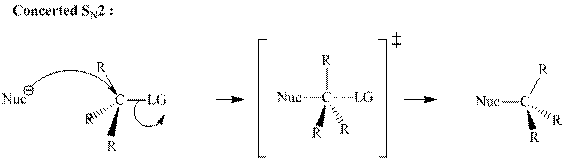
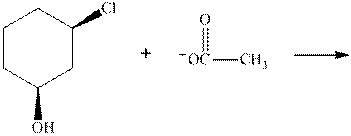
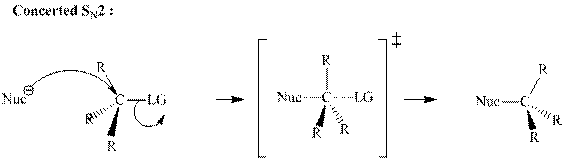
(d)
Interpretation:The mechanism by curved-arrow notation for below reaction should be written.
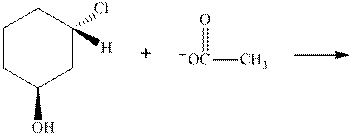
Concept introduction:Bimolecular substitution or
A general
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic Chemistry: Structure and Function
- a) Consider the reaction of HBr with ethylene and propylene. At roomtemperature the reaction of propylene with HBr is much faster than thereaction with ethylene.Using reaction energy diagrams and your knowledge of carbocationstability explain why this is so. b) Xylene (dimethylbenzene) is a commonly used chemical in the printingindustry and as a cleaning solvent for oily waste. It is also used whenpreparing histological samples to remove waxes from biological samples.Draw the three possible structures for this compound and give the UPACnames for each. Define which structures are ortho, meta, and para.arrow_forwardCan someone draw out the mechanism for this reaction?arrow_forwardWrite the products for (A-I). Label products with the mechanism (Sn2, Sn1, E1, E2) that produced it and write out and label major vs minor elimination products.arrow_forward
- 6. Reactions at the a Carbon - Mechanism reaction below. Provide a reasonable mechanism for the H* / H2O طلع بس مالarrow_forwardExplain how you can tell from the energy diagram that the reaction with the catalyst in Fig. 8.4 isfaster than the reaction without the catalyst.arrow_forwardA problem often encountered in the oxidation of primary alcohols to acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate: Propose a mechanism to account for the formation of ethyl acetate. Take into account the reversible reaction between aldehydes and alcohols:arrow_forward
- Give one example of elimination reaction (E2) type. Show the reactants materials and the product. Draw the reaction mechanism?arrow_forwardCarry out using the appropriate mechanisms for the reactions given below. Indicate the name of the mechanism used.arrow_forwardWrite product and mechanismarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning




