
Concept explainers
(a)
Interpretation:Part of the sanjoinine molecule that is derived structurally from
Concept introduction:Peptides are defined as smaller fragment of amino acids that are linked by special peptide linkage that is essentially an amide
(b)
Interpretation: Four diastereomers of
Concept introduction: Bimolecular substitution or
A general
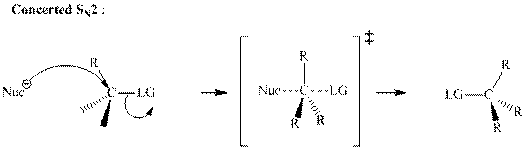
Polar-aprotic solvents accelerate the rate of
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic Chemistry: Structure and Function
- Hypoglycin A, an amino acid derivative found in unripened lychee, is anacutely toxic compound that produces seizures, coma, and sometimesdeath in undernourished children when ingested on an empty stomach. (a) Draw the neutral, positively charged, and negatively charged forms of hypoglycin A. (b) Which form predominates at pH = 1, 6, and 11? (c) What is the structure of hypoclycin A at its isoelectric point?arrow_forwardC) a nitrile D) an amine nitrite salt DIRECTIONS: Each of the following questions or incomplete statements below is followed by four suggested answers lettered A - D. Select or choose the letters of the best answer to the question or incomplete statement. 7. An organic nitrogen compound, X, gives ammonia on warming with dilute aqueous sodium hydroxide, X could be A) ethanamide B) ethylamine C) phenylamine D) amino ethanoic acid Which one of the following reagents reacts 1. in a similar fashion with both phenylamine (C H,NH,) and ethylamine. A) Br,(aq) C) conc. HNO, D) cold HNO (aq) 2. Phenylamine (aniline) can be prepared by reducing nitrobenzene with tin and concerntrated hydrochloric acid followed by the addition of alkali and finally steam distillation. The alkali is added to; A) prevent oxidation of phenylamine. B) liberate free phenylamine from solution C) dissolve excess nitro benzene D) dissolve the phenylamine 8. Arrange the following molecules in their increasing order of base…arrow_forwardPlease complete the following question. There is no need to provide an explaination. 2arrow_forward
- Give reasons: (i) Bond length of C = O in carboxylic acids is slightly larger than C = O bond length in carbonyl compounds. (ii) There are two –NH2 groups in semicarbazide. However, only one –NH2 group is involved in the formation of semicarbazones. (iii) Benzoic acid is less soluble in water than acetic acid. (iv) Formic acid is a stronger acid than acetic acid.arrow_forwardProteases are enzymes that can break covalent bonds in proteins. Proteases play major roles in the regulation of biological processes, so compounds that inhibit their function, called protease inhibitors, have potential as therapeutic agents. While preparing several potential protease inhibitors, compound 1 was converted into compound 3 via alkyne 2, as shown. (J. Org. Chem. 1989, 54, 3963- 3972) Draw the structure of alkyne 2, and propose reagents for converting 1 into 3. Step 1 Br Br Ph alle Br Br Edit Drawing OH Modify the given structure of 1 to draw alkyne 2. An eraser is available, and you can use the single bond tool to add/remove pi bonds. OH 2 Ph O ell (-10) Ph = 3 OHarrow_forwardWhich of the follwoing compounds is responsible for the mass sepctrum and why? (a) octanol, CH3CH2CH2CH2CH2CH2CH2CH2OH (b) heptane, CH3CH2CH2CH2CH2CH2CH3 (c) acetic acid, CH3COOHarrow_forward
- ive the structure and the correct IUPAC name for a compound that is representative of each of the following: (i) Acetal (ii) Alkoxide salt (iii) Cyanohydrin (iv) Secondary Amine (v) Tertiary Alcoholarrow_forwardchoose the more basic compound and give your explanation.arrow_forward(B) Choose the more acidic member of the following pair of isomers. Justify your answer. HO. HO. 어 H or Harrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
