
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
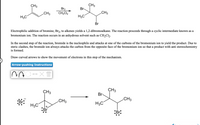
Transcribed Image Text:CH3
CH3
Br-
Br2
CH2CI2
CH3
CH3
H3C
H3C
Br
Electrophilic addition of bromine, Br2, to alkenes yields a 1,2-dibromoalkane. The reaction proceeds through a cyclic intermediate known as a
bromonium ion. The reaction occurs in an anhydrous solvent such as CH2C12.
In the second step of the reaction, bromide is the nucleophile and attacks at one of the carbons of the bromonium ion to yield the product. Due to
steric clashes, the bromide ion always attacks the carbon from the opposite face of the bromonium ion so that a product with anti stereochemistry
is formed.
Draw curved arrows to show the movement of electrons in this step of the mechanism.
Arrow-pushing Instructions
CH3
CH3
Br-
.CH3
.CH3
H3C
H3C
:Br
:Br:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Suppose you were told that each reaction is a substitution reaction, but you were not told the mechanism. Describe how you could conclude from the structure of the haloalkane or cycloalkene, the nucleophile, and the solvent that each reaction is an SN1 reaction.arrow_forwardSuppose you were told that each reaction is a substitution reaction, but you were not told the mechanism. Describe how you could conclude from the structure of the haloalkane or cycloalkene, the nucleophile, and the solvent that each reaction is an SN1 reaction.arrow_forward10. Choose the major organic product of the reaction. A | В II IV Br Br Br Br Br Br2 Br Br 100°C Br IV =arrow_forward
- Draw curved arrows to show the movement of electrons in this step of the mechanism.arrow_forwardDraw the energy diagram of the following reaction (label and draw reactants, intermediates, and products). Label transition state of the rate limiting step and draw the transition state of the rate determining step. Is it a concerted or stepwise reaction? Write the rate law for this reaction. How do we increase the reaction rate? If we use deutrium-tert-butyl bromide such as (CD3)3CBr instead of (CH3)3CBr, will it increase or decrease the reaction rate? Or there is no meaningful rate change. Write reasons for your answer. Draw mechanisms for reactions by drawing arrows and intermediates.arrow_forwardConsider the mechanism for the given nucleophilic substitution reaction. X 2H₂0arrow_forward
- Write the expected substitution product(s) for each reaction and predict the mechanism by which each product is formed.arrow_forwardConsider the reaction of NaCl with 2- bromo-2-methylhexane in acetone/water. Predict the change in rate if the concentrations of both the 2-bromo-2- methylhexane and the NaCl are doubled. A) The reaction rate stays the same. B) The reaction rate doubles. C) The reaction rate is halved. D) The reaction rate quadruples E) The reaction rate triples.arrow_forwardDraw the structure of the four allylic halides formed when 3-methylcyclohexene undergoes allylic halogenation with NBS + hv.arrow_forward
- Draw a structural formula for the major organic product of each reaction and specify the most likely mechanism by which each is formed.arrow_forwardEthers can be prepared by reaction of an alkoxide or phenoxide ion with a primary alkyl halide. Draw the structure of the expected organic product of the reaction of iodomethane with the following alkoxide ion: CH3 H3C O Na You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. • Do not include lone pairs in your answer. They will not be considered in the grading. • Do not include counter-ions, e.g., Na", I, in your answer. орy вste ChemDoodlearrow_forwardWhat is the NAME of the Mechanism for this reaction?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY